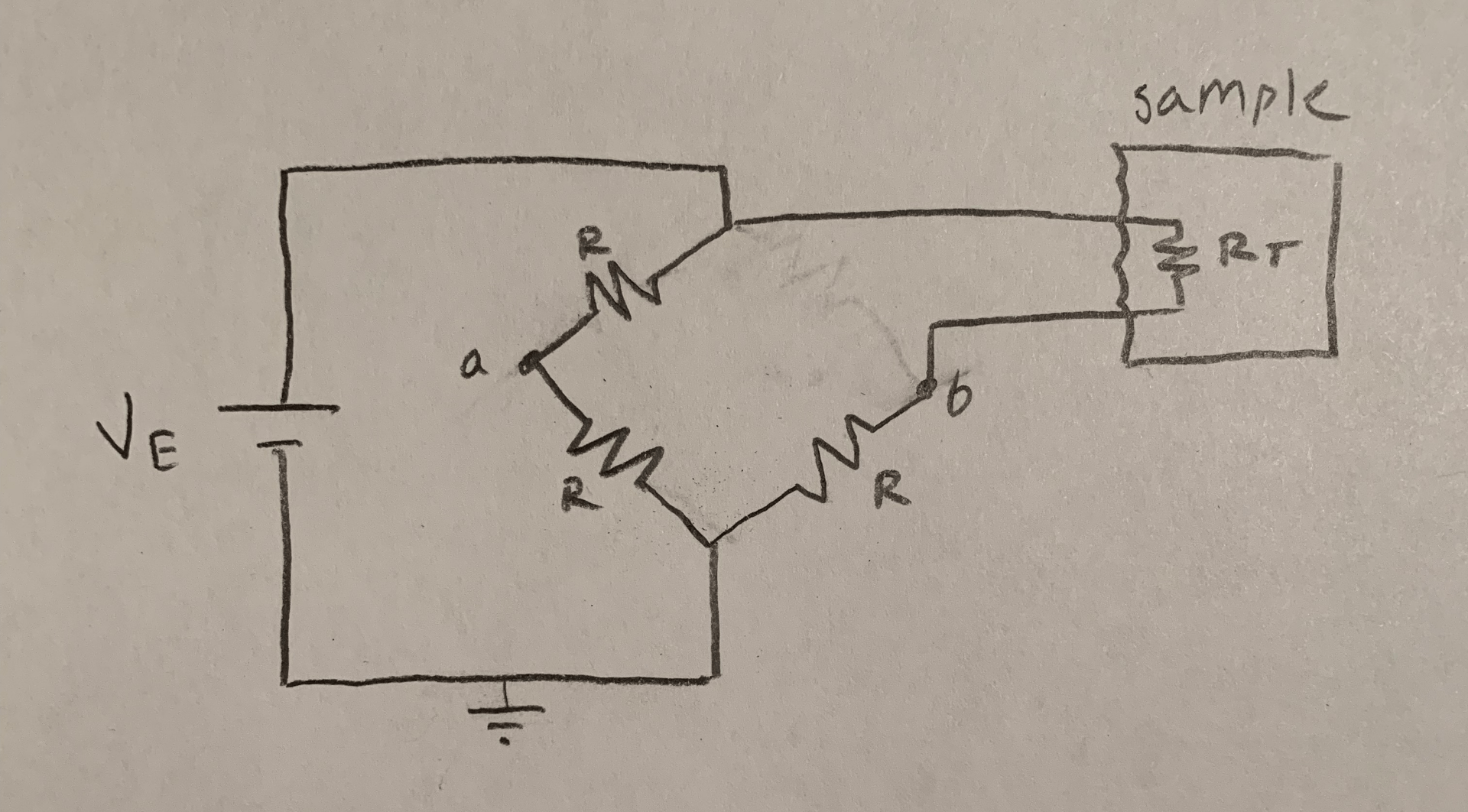
PROBLEM
In order for whole body air displacement plethysmographic machines such as the BodPod to function optimally (so that viable data can be collected), it is crucial that laminar flow is maintained throughout the machine’s ventilation system at all times. Imagine that you are an engineer (imagine that!) tasked with manufacturing the tube components for the Bod Pod.
If the flow rates in the inlet and outlet tubes are equal, the volumetric flow rate of air in the tubing system will be 0.25 cubic meters/second , and the BodPod functions in laminar/laminar-like conditions, what are the ideal dimensions for the diameters of the inlet and outlet tubes in the Bod Pod?
Assumptions
- Flow rates are equal in the inlet and outlet tubes
- The tubes are cylindrical
- Laminar flow is maintained at all times
- Pressure changes are negligible
- Air circulating inside the BodPod has similar thermodynamic/kinematic properties ambient air at room temperature
- Temperature conditions of the device are identical to those at room-temperature
A link has been included to a power point presentation that contains diagrams that will aid readers in solving this problem:
https://docs.google.com/presentation/d/178RDbb09Vjwv3o7JVS9bkwR1TMjgWWApOBE5WDRzaQE/edit#slide=id.p

Figure 1: Schematic of Adult-Sized Bod Pod and circuitry components that will be used as a reference for this problem.
BACKGROUND KNOWLEDGE/ ASSUMPTIONS
According to the 4th page of the patent filed by the manufacturer, Life Instruments Inc., it is okay to assume laminar conditions inside the tubing ventilation due to the fact that flow rate inside the inlet and outlet tubes are always set to values of low magnitudes. Literature in courses such as Signals and Systems show that low flow rates result in low generation of acoustic noise by air circulation systems.
I was unsuccessful in locating some sort of testing standard that establishes set values for the volumetric flow rates of air in laminar conditions. There appears to be any information pertaining to any testing protocols the manufacturer used for design verification purposes in the original 510(k) form filed with the FDA. To establish an appropriate flow rate value for this test question, I searched for similar problems online. In short, the values for the volumetric flow rate of air (Q) ranged from 0.1 to 0.8 cubic meters/second in my searches. I decided to use a value of 0.25 cubic meters/second in this problem. By assuming that the values for Q are equal for both tubes, it is possible to design both tubes with an equal diameter. Thus, along with other reasons that will be outlined later in this section, all the solver is required to do to calculate the correct value in this problem is to use one equation.
Normally, pressure fluctuations trigger changes in tubings and pipes create flow gradients in closed ventilation systems. Because of this, mathematical expressions such a Boyle’s Law and Bernoulli’s equations are used to solve changes in volume and volumetric flow when pressure fluctuations occur. According to page 4 of the patent filed for the Bod Pod, the authors state that the use of pressure transducers which are coupled to the inlet and outlet tubes helps monitor any pressure changes that occurs in the tubing; automatically adjusting the pressure settings in the tubes to more optimal levels through negative feedback. This is done in order to maintain a constant flow rate (and thus, laminar flow throughout the circulation system). Later on in section 4 of the patent, the manufacturers also state that constant air flow can be maintained with the addition of rotary pumps to the circulation system (which are not actively displayed in any of the figures included).
The manufacturer’s statements in the patent confirm the presence of temperature-sensing circuitry in the inlet and outlet tubes that control the internal temperature of the environment inside the tubing and the pod itself. Thus, any temperature fluctuations that could create flow gradients in the device’s tubing are negligible since they are always corrected in rapid fashion. This also eliminates the need for Fourier’s law to solve the value of Q in this problem.
Assuming that the tubing is cylindrical eliminates the need to solve for any hydrodynamic radius values(which are used in equations associated with fluid flow in which tubes/pipes are any shape that is non-cylindrical).
By assuming that the air inside the device’s circulation system behaves in a similar fashion to ambient air, and that the conditions inside the circulation system are similar to those at room-temperature and that the device is used in STP conditions, it is possible to estimate the value of the kinematic viscosity of air (which is needed to solve the value for the diameter of the tubing using the Reynolds number equation along with the value of the flow rate given in the problem description and the upper-limit value of the Reynolds number associated with laminar flow).
SOLUTION
In order to solve for the value of the tube diameter, the solver must utilize the following equation:
Re = QD/v ,
Re = reynolds number
Q = volumetric flow rate of air
D = pipe bore or tube diameter
v = kinematic viscosity
NOTE: Pipe bore is equivalent to the diameter of the tube, and this equation is applicable to both pipe and duct installations.
First, the value of Q is already provided in the description. So the reader is already provided one unknown.
Second, the reader is told in the problem description and background section to assume laminar conditions in the circulation system. The Reynolds number value used in this problem is 2300, which is the established upper limit for laminar flow. All values at or below this number is considered laminar flow.
Third, since the reader is told to assume that the air circulating through the inlet and outlet tubes are similar in kinematic/thermodynamic behavior to ambient air at room temperature, the reader can assume that air inside the circulation system has the same kinematic viscosity as ambient air at room temperature. This value is 1.494 x 10-5 meters ^2/ second.
At this point, the only unknown that the reader is left with is the value of D, or the tube diameter. After plugging all the known values into the above-aforementioned equation and solving for the value of D algebraically, the reader should arrive at a diameter value of approximately 0.13708 meters.
REFERENCES
[1] Dempster Phillip, Michael Homer, and Mark Lowe (2004). United States Patent 20040193074A1. Retrieved from https://patentimages.storage.googleapis.com/93/cf/ea/6d2d1346ea1129/US20040193074A1.pdf
[2] Engineers Edge. “Kinematic Viscosity Table Chart of Liquids” (2019). Machinery’s Handbook, 29th edition. Retrieved from
https://www.engineersedge.com/fluid_flow/kinematic-viscosity-table.htm
[3] Foster, Trevon. “Laboratory Flow Meters: Flow Measurements In the Lab” (2015). Titan Enterprises, Ltd. Retrieved from
https://www.flowmeters.co.uk/laboratory-flow-meters-flow-measurement-in-the-lab/