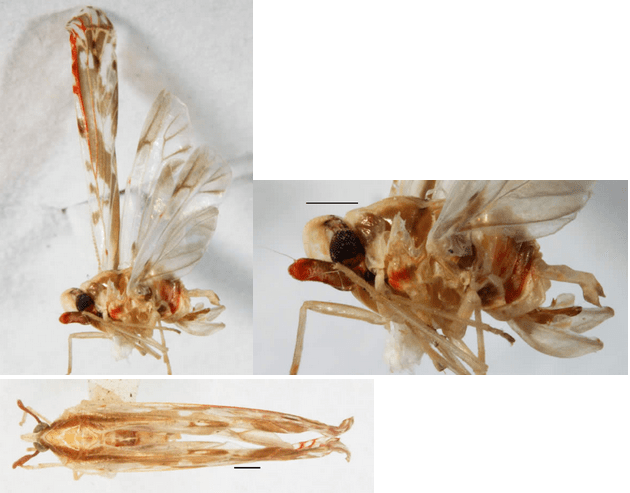
[Back to North American Derbidae]
Contents
- 1 Family Derbidae Spinola, 1839
- 1.0.1 Subfamily Otiocerinae Muir 1917
- 1.0.2 Tribe Otiocerini Muir, 1917
- 1.0.3 Genus Anotia Kirby, 1821
- 1.0.4 Distribution
- 1.0.5 Recognized species
- 1.0.6 Economic Importance
- 1.0.7 Plant associations
- 1.0.8 Recognition
- 1.0.9 Online resources
- 1.0.10 Collecting
- 1.0.11 Molecular resources
- 1.0.12 Selected references
Family Derbidae Spinola, 1839
Subfamily Otiocerinae Muir 1917
Tribe Otiocerini Muir, 1917
Genus Anotia Kirby, 1821
Type species Anotia bonnetii Kirby, 1821.
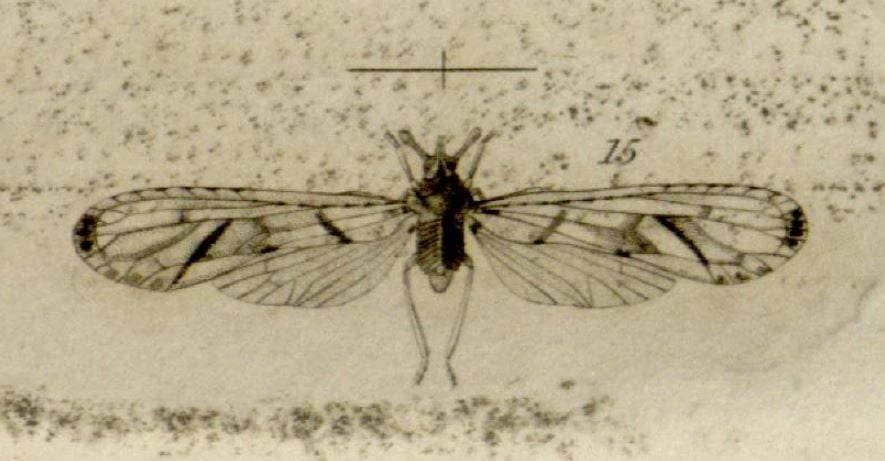
Kirby’s 1821 depiction of Anotia bonnetii
Synonyms
Amalopota Van Duzee, 1889: 176 (Type species Amalopota uhleri Van Duzee 1889); syn. by Fennah 1952: 152.
Van Duzee 1889: 177 noted, “This genus differs from Anotia, to which it is perhaps most nearly related, by the presence of ocelli, the greater length of the rostrum, the smaller number of veins in the stigmatal region, etc.”
Fennah 1952: 152 “With series of varied Anotia before him the writer [Fennah] found intermediate stages in characters which serve to distinguish Anotia bonnetii Kby. from Amalopota uhleri Van Duzee, and accordingly cannot at present recognize Amalopota as a separate genus, or even as a subgenus.”
This statement has been taken to mean that Amalopota is a synonym of Anotia, but Fennah did not make a formal statement of synonymy -viz. listing Amalopota as a syn. of Anotia on p. 157 of the same work, which may have led to subsequent confusion. Perhaps the matter should be reviewed, although the key diagnostic differences (e.g., those given by Metcalf 1923 in couplet 185 in the screenshot below) seem to me unconvincing, or at least incomplete, as genus-level features.
Distribution
Widespread in the Nearctic (esp. south) and Central America.
Recognized species
By my count, 22 species in the Nearctic and Central America.
Amalopota in Metcalf 1945: 142.
Anotia in Metcalf 1945: 144.
Anotia bonnetii Kirby, 1821: 21 – USA: CT, FL, GA, IL, KS, NC, NJ, NY, OH, TX; CAN: ON; Mexico (Guerrero)
= Anotia ruficollis Fowler, 1904: 78; syn. by Ball 1928: 197.
Anotia burnetii Fitch, 1856: 395 – USA: IL, IN, MS, NC, NY, PA, TX; CAN: ON
Anotia caliginosa Ball, 1937: 182 – USA: AZ
Anotia cerebro Bahder & Bartlett, 2023 – Costa Rica
Anotia fitchi (Van Duzee, 1893) – USA: FL, GA, IL, KS, MO, MS, NC, NY, OH, OK, PA, SC, TN; Cuba; Mexico (Tabasco); Panama
= Amalopota fitchi Van Duzee, 1893: 280.
= Anotia fitchi (Van Duzee, 1893); comb. by implication Fennah 1952: 152.
= Anotia venustula Fowler, 1904: 78; syn. by Ball, 1928: 197.
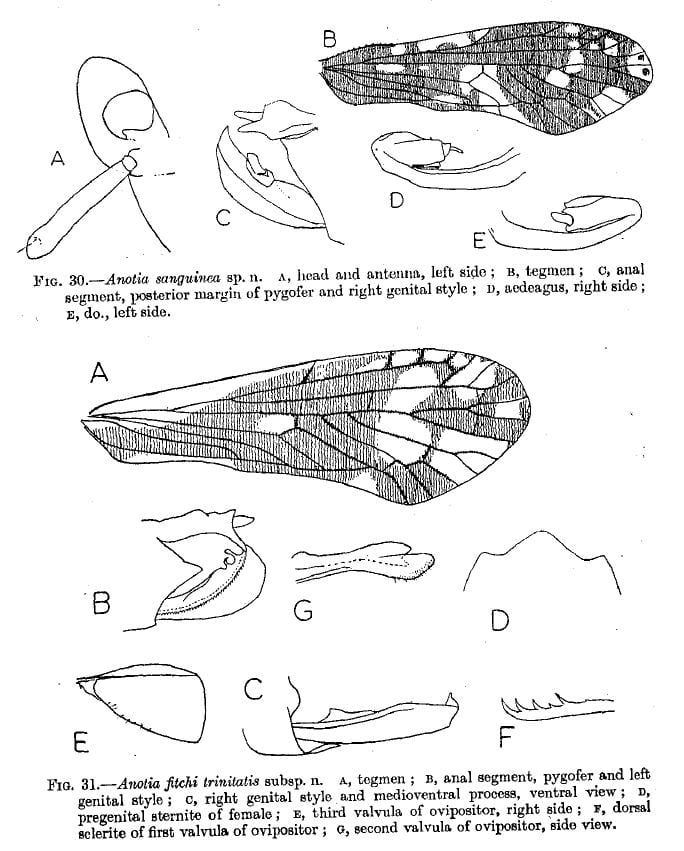
= Anotia fitchi trinitatis Fennah, 1952: 159 – Trinidad
Anotia firebugia Bahder & Bartlett, 2020 – Costa Rica (Alajuela) (this species has shown up also in Limón Province, Costa Rica; USA: Texas (Nueces, Fort Bend, Austin, Cameron and Hildalgo Counties), Nicaragua, and Belize.
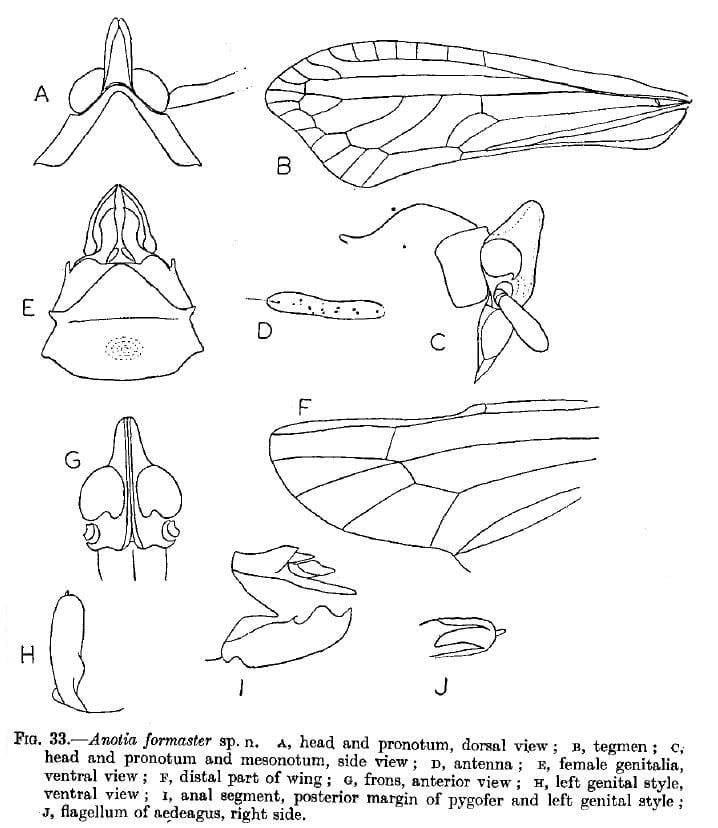
Anotia formaster Fennah, 1952: 160 – Trinidad
Anotia invalida Fowler, 1904: 79 – Guatemala, Panama
Anotia kirkaldyi Ball, 1902b: 259 – USA: DE, IA, IL, KS, MS, NC, OH, PA, VA; CAN: ON
Anotia lineata Ball, 1937: 183 – USA: AZ
Anotia mcateei (Dozier, 1928) – USA: IL, MS
= Amalopota mcateei Dozier, 1928: 141-142.
= Anotia mcateei (Dozier, 1928); comb. by implication Fennah 1952: 152.
Anotia marginicornis Fowler, 1904: 77 – Guatemala
Anotia pellucida Fowler, 1904: 77 – Mexico (Veracruz, as “Orizaba”)
Anotia posa DeHass, Bahder & Bartlett, 2025 – Bonaire, Curaçao
Anotia punctata Metcalf, 1938: 322 – Panama
Anotia robertsonii Fitch, 1856: 395 – USA: AR, DE, FL, NC, NJ, OK, PA
Anotia rubrinoda Fennah, 1952: 159 – Trinidad
Anotia sanguinea Fennah, 1952: 157 – Trinidad
Anotia smithi Fowler, 1904: 77 – Mexico (Guerrero)
Anotia tenella Fowler, 1904: 78 – Mexico (Guerrero)
Anotia uhleri (Van Duzee, 1889: 176) – USA: FL, IL, IN, MN, MO, MS, NC, NY, OH, RI, TN; CAN: ON, QC
= Amalopota uhleri Van Duzee, 1889: 178.
= Anotia uhleri (Van Duzee, 1889); comb. by implication Fennah 1952: 152.
Anotia westwoodi Fitch, 1856: 934 – USA: DC, DE, IL, KS, MD, MO, NC, NJ, NY, OH, PA, TN; CAN: ON
Note:
Interamma septentrionalis Anufriev, 1968 (from the Russian far east, evidently Korea also) was moved to Amalopota septentrionalis (Anufriev, 1968) by Emeljanov 1999 (full citation below). The placement of this species in the invalid genus Amalopota needs review, particularly since it implies that Anotia should include Old World members.
Economic Importance
Limited.
Plant associations
Derbidae are known or assumed to feed on fungal hyphae as immatures. The significance of adult host associations is unclear.
- Anotia bonnetii – Salix (Fitch 1893: 387 [reprint of 1851])
- Anotia burnetii – French mulberry (American beautyberry) (Callicarpa americana L., Verbenaceae), ironwood (American hornbeam), (Carpinus caroliniana Walter, Betulaceae) (Dozier 1928)
- Anotia firebugia – Cocos nucifera (coconut, Arecaceae.) (Barrantes et al. 2020)
- Anotia fitchi – Saccharum officinarum L. (sugarcane), Spartina pectinata Bosc ex Link (prairie cordgrass, Poaceae), Carya (Hickory, Juglandaceae)
- Anotia robertsonii – Fraxinus sp. (Ash), reported here, and here (underside of leaves; both observations iNaturalist)
- Anotia uhleri – Acer (Maple, Aceraceae), ‘Five specimens of Amaloptera uhleri Van D. were captured from hickory. This species is sometimes found on dogwood’ (Spooner 1937); and Ash (iNat), and oak (iNat)
- Anotia westwoodi – “…grass and on willows in lowland meadows, from the beginning of August till the end of the season…” (Fitch 1856: 395)
- Anotia sp. – Senna obtusifolia (L.) Irwin & Barneby (Java-bean. sicklepod, Caesalpiniaceae) (Palmer & Pullen 2001)
Hosts from Wilson et al. 1994 or as cited; plant names from USDA PLANTS or Tropicos.
Recognition
Pale, fragile forms; wings greatly exceeding the abdomen; frons extremely compressed; pustules along claval vein; clavus open, antennae without appendages (‘Anotia‘ means absence of the external ear’), head projecting in front of eyes for distance similar to width of eyes, head roundly triangularly pointed up at 45 degree angle, costal margin of wing without appendage (vs. Sayiana).
Species in this genus are recognized largely by the color pattern of the wings and head. This can present challenges because the color pattern is not fully developed in freshly emerged (i.e., teneral) adults (and the color fades in old specimens), and it is not entirely clear how much the color pattern varies in adults. Also, to date, genitalia have not been described for any of these species, so it is possible that not all described species north of Mexico are ‘good’ species, or that there are species in addition to those currently described. Some species are incompletely characterized. Neotropical species are undoubtedly more diverse than currently described (for example, Costa Rican species photographed by Gernot Kunz).
Eastern species can be identified in Bartlett et al. 2011.
Amalapota was described in surprising detail in Van Duzee 1889: 176, provided below; in a footnote, Van Duzee noted that Amalapota came from Greek meaning “feeble flight”. [corrected OCR from BHL, although there may still be errors]
Form very slight. Head rather short, horizontal above, almost vertical before, with superior and frontal keels about as in Anotia; apex obtuse. Eyes of medium size, emarginate below. Ocelli two, distinct, placed below and very near the inferior angles of the eyes. Antennae about as long as the head, situated at the base of the clypeus in a socket formed by a sharp, slightly elevated ring ; basal joint very short and annular; second joint diverse in the two sexes; in the male, much flattened, with the sides almost parallel ; in the female, shorter and slightly flattened ; in both sexes papillated, with a subterminal emargination, from which springs a bristle. Clypeus triangularly ovate, convex. Rostrum long, reaching to about the middle of the venter; terminal joint very short. Prothorax linear above, produced in an acute angle between the eyes ; on the sides, suddenly expanded to a broad, thin scale. Humeral scales large and prominent. Legs slender, unarmed, of medium length; the posterior femorae somewhat thickened; posterior tarsi three jointed basal joint longer than the second and third united. Elytra long and narrow, widest at the inner apical angle ; apex broadly rounded, a little retreating posteriorly; the costal area expanded near the base into a broadly rounded, slightly recurved lobe ; a slight constriction of the costa just before the apex, with a thickening of the veins there, produces the appearance of an imperfect stigma. Venation simple, almost as in Anotia; costal area rather broad ; mediastinal vein forked at the basal third; costal branch sending about two veinlets to the costa in the stigmatal region, and united by a cross vein to the outer fork of the inner branch, which is straight and twice forked just before the apex. Postcostal vein joining the mediastinal near the base and running straight 10 the apex of the elytra, parallel to the inner branch of the mediastinal vein; the long, straight cell thus formed is crossed by two veinlets, one at the apical third, the other near the apex. A cross vein joins the post-costal with the median vein near the middle of the elytra, beyond which the former sends five branches to the inner apical margin, the basal two of which are themselves forked near their apex, and united by a zigzag submarginal vein that reaches the claval suture ; at this submarginal vein terminate the anal and the two branches of the median vein. The apical forks of the post-costal vein are united by slender cross veins, which with this submarginal vein form a series of about twelve apical and marginal areoles from the semi-stigma to the clavus. Wing : — Mediastinal vein simple, near the costa, which it touches at about the middle ; post-costal vein bifid before the apex, and united by a cross vein to the mediastinal and median veins, the latter of which is also bifid. Abdomen short and broad, with a dorsal carina; showing five segments above and four beneath.
The vertex and front are so compressed into the superior and frontal keels that they might not improperly be described as wanting. These keels, as in Anotia are united on the front and divergent posteriorly on the vertex, the included space being cut out to receive the pronotum. The mesonotum is convex and lozenge-shaped, the length scarcely greater than the width, which greatly exceeds that of the head ; with three dorsal carinae. Four anterior coxae long and slender, placed obliquely; posterior short and thick. Base of the femorae approximate. The genital pieces scarcely differ from those of Otiocerus.
This genus differs from Anotia, to which it is perhaps most nearly related, by the presence of ocelli, the greater length of the rostrum, the smaller number of veins in the stigmatal region, etc.; from Patara by the presence of ocelli, the greater length of the rostrum, the shape of the head and thorax, and the venation; from Mysidia and Derbe (Westw.), it differs in the single frontal carina, in the shape and venation of the wings and the form of the eyes, but agrees with the latter genus in the presence of the costal constriction (although less pronounced), and the length of the rostrum. The only genus described by Stal to which it needs to be compared is Hulcita, from which it is sufficiently differentiated by the presence of ocelli, the form of the vertex, antennae, etc.
Key to eastern species from Metcalf (1923). Amalapota is a synonym of Anotia. Couplet 188 does not seem to work well since westwoodi & kirkaldyi also can have spots.
I am going to briefly describe some impressions of North American Anotia species. Because the genus is common, I have often been asked to identify specimens – particularly from photos – and some species definitions are unclear. This is in part because of variation (including that recently emerged individuals appear to e paler), and in part because species definitions are dependent entirely upon color without being confirmed by genital features (not described for most species).
The species Anotia fitchi, Anotia uhleri, Anotia mcateei, and Anotia lineata appear to me to be fairly distinctively marked and are commented on below. The 6 remaining species have a wing pattern where dark markings predominantly follow the forewing veins. If I am not mistaken – and I may be – in Anotia robertsonii the vein markings are intense in the middle of the distal region of the remigium on what appear to be M-veins., and contrasting with an otherwise paler wing. In Anotia burnetii (where I have not seen enough specimens to be sure, this one, and this one, might be burnetti), the same pattern is evident as robertsonii, but less intense. Anotia caliginosa is the only western species with the dark markings following the veins (to me, the wing markings appear pallid and narrow, although slightly more intense on the same veins as robertsonii).
The remaining species – Anotia bonnetii, Anotia kirkaldyi and Anotia westwoodi appear similar in many respects (caliginosa also, if distribution is discounted), and I have had difficulties finding consistent differences; bonnetii is supposed to have round markings near the cells on the distal margin of the wings, and kirkaldyi has some veins notably darkened. However, I suspect that all three species have rounded spots in the cells near the apex of the wing (how clear these are is another matter), and the dark vein issue, although clear sometimes, is hard to judge.
In combination with the wing features, I have been using the color features of the head, which seem consistent but vary in intensity. If it holds, it appears that kirkaldyi has a pair of reddish markings on the head; in contrast, westwoodi has a blackish marking on the top of the head (lateral view), like this:
This leaves bonnetii, which remains a problem to me; bonnetii may either be a pallid species (with 1 red head marking, like the specimen below), or an intensely marked species with 2 red head markings, differing from kirkaldyi in having no dark veins (and qualitatively different spots at the wing apex). Most of the specimens recorded as bonnetii e.g. on bugguide, I would want to call kirkaldyi, but the lack of clarity between the species, and uncertainty about the degree to which color features are consistent among species, has prevented me from providing any clear diagnostic guidance until I (or someone) studies the tails.
Still, there are images of some specimens (e.g., here) that are not clearly any of the above. [BTW, my guess is that one is the real Anotia bonnetii.
I am really puzzled with respect to what the ‘real’ Anotia bonnetii is; most of the images I see called bonnetii are what I would call kirkaldyi. (I think I have them sorted out now, I hope to publish on the issue in 2021).
Kirby’s (1821) image:

Anotia bonnetii from Kirby 1821.
This image reminds me of most of what I call Anoria robertsoni, viz.

Anotia robertsoni (iNaturalist by Judy Gallagher, Woodbridge, VA)
But some are more pallid, like [bugguide] or [bugguide] (I’ll see if I can find one to post)
Anotia burnetii Fitch, 1856 – characterized by middorsal dark markings on first 3 abdominal segments. I have seen very few specimens that I can refer to this species. I note that the terga of Anotia may be pale or marked either with dark (‘black’) or red (but apparently only burnetii has them on the post proximal abdominal terga). It is unclear to me whether such abdominal markings are consistent among species. . Spooner (1937) reports 9 specimens collected in southern Illinois (in August; perhaps the species is mostly Midwestern).
My guess is that Anotia burnetii in life looks like this [bugguide ] and [ bugguide] and [bugguide] and [iNaturalist]

Anotia caliginosa (Salvador Vitanza, Nogales, Santa Cruz County, Arizona, 31.335262, -110.936444, 3,936 ft, August 31, 2017, mercury vapor and ultraviolet lights)
Anotia fitchi (Van Duzee, 1893) – a former Amolopota, recognizable for red markings on the body and wings; wings are broadly marked with dark; markings on the mesothorax suggest lineata.
Anotia lineata Ball, 1937 – one of only 2 predominately western species, lineata can be recognized by its color pattern suggesting pair of dark vitta beginning on the gena, extending below the eyes, across the lateral aspects of the pronotum and forewings, becoming broader and more diffuse on the wings.
In life like this [bugguide] or [bugguide]
Anotia mcateei

Anotia mcateei from iNaturalist [Perry Co., Ohio]
Anotia mcateei (Dozier, 1928) from Dozier (1928) (Dozier’s types are missing)
A good example in life is [Hoppers of NC] or [bugguide]
Anotia uhleri (Van Duzee, 1889: 176) – the type species of Amalopota, the only species with entirely red-themed patterning, including most of the body (not the legs), the base and apex of the forewing, leaving the middle region of the forewings clear.
A lot of in vivo images, like [bugguide] and [hoppers of NC]
I haven’t seen a good example of this species online, which is odd.

This appears to be an Anotia to me, unless I am missing the projection on the costal margin of the wing and it is Sayiana (photo by Mark de Silva from the Island of Mustique in the Grenadines); not sure if this is a described species.

Portion of Fowler (1904) plate 9, species (as given in Fowler) 1. Otiocerus breviceps, 2. Otiocerus rubescens, 3. Anotia smithi, 4. Anotia marginicornis, 5. Anotia ruficollis, 6. Anotia venustula, 7. Anotia tenella, 8. Anotia invalida, 9 Patara marmorata, 10 Ramphixius championi, 11. Bothriocera tinealis, 12. Bothriocera tinealis v. westwoodi.
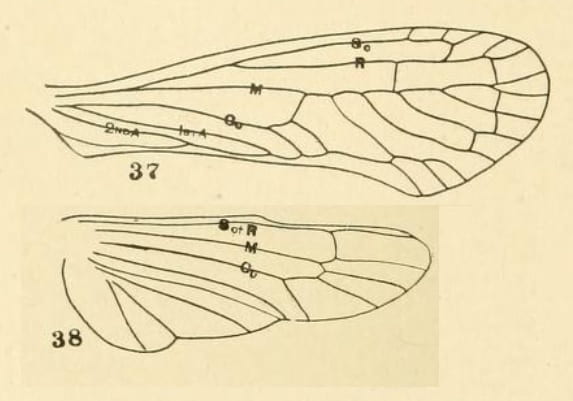
Anotia sp. wings venation from Metcalf 1913.
Online resources
TaxonPages. World Auchenorrhyncha Database
bugguide.
FLOW.
BOLD.
EOL.
iNaturalist.
GBIF.
El Paso County extension education.
Hoppers of North Carolina.
Fulgores Liste des espèces Fulgoroidea Hémiptères.
Maryland biodiversity.
Discover Life.
Collecting
Found infrequently at lights or by sweeping, especially in cool, wet situations (and beneath the leaves of broad-leaved plants in damp or mesic areas). Adults are assumed to remain near the habitat for immatures.
Molecular resources
Genbank has data for four species as of this writing. BOLD database has 21 specimens representing 4+ species.
Selected references
Anufriev, G.A. 1968. Cicads of the family Derbidae (Homoptera, Auchenorrhyncha) in the fauna of the USSR. Entomologicheskoe Obozrenie 47(1): 133–1146.
Ashmead, W.H. 1889b. A generic synopsis of the Fulgoridae (Concluded.). Entomologica Americana 5(2): 21-28.
Bahder, B.W., M.A.Z. Echavarria, E.A.B. Barrantes, E.E. Helmick, G. Kunz & C.R. Bartlett. 2023. A new species of planthopper in the genus Anotia (Hemiptera: Auchenorrhyncha: Derbidae) from the Los Angeles cloud forest in Costa Rica. Zootaxa 5380(2): 184–194. https://doi.org/10.11646/zootaxa.5380.2.6
Ball, E.D. 1902. New genera and species of North American Fulgoridae. Canadian Entomologist 34: 259-266.
Ball, E.D. 1937. Some new Fulgoridae from Western United States. Bulletin of the Brooklyn Entomological Society 32: 171-183.
Barrantes, E.A., M.A. Zumbado Echavarria, C.R. Bartlett, E.E. Helmick, P. Cummins, M.S. Ascunce & B.W. Bahder. 2020. A new species of planthopper in the genus Anotia Kirby (Hemiptera: Auchenorrhyncha: Derbidae) from coconut palm in Costa Rica. Zootaxa 4763 (1): 50–60. https://doi.org/10.11646/zootaxa.4763.1.4
Bartlett, C.R. & J.L. Bowman. 2004. Preliminary Inventory of the Planthoppers (Hemiptera: Fulgoroidea) of the Great Smoky Mountains National Park, North Carolina and Tennessee, U.S.A. Entomological News 114(5): 246-254.
Bartlett, C.R., E.R. Adams & A.T. Gonzon. 2011. Planthoppers of Delaware (Hemiptera, Fulgoroidea), excluding Delphacidae, with species incidence from adjacent States. ZooKeys 83: 1–142.
Bartlett, C. R., L.B. O’Brien & S.W. Wilson. 2014. A review of the planthoppers (Hemiptera: Fulgoroidea) of the United States. Memoirs of the American Entomological Society 50: 1–1287.
Benton, E.P. & J.W. McCreadie. 2009. A preliminary survey of the planthoppers (Hemiptera: Fulgoroidea) of coastal Alabama. Proceedings of the Entomological Society of Washington 111(2): 354–1360.
Bory de Saint-Vincent, M. (Jean Baptiste Geneviève Marcellin). 1823. Cicadaires. Pp. 109-111. In: Dictionnaire classique d’histoire naturelle, Tome Quatrieme. CHI-COZ.. Paris,Rey et Gravier, Libraries-Editeurs, Auai des Augustins, no. 55; Baudouin Frèrer, Libraries-Editeurs, Imprimeurs de la société D’Histoire Naturelle, Rue de Vaugirard, no. 36,1822-31. 628 pp. [genus noted]
Britton, W.E. 1920. Check-list of the insects of Connecticut. Bulletin of the Connecticut Geological and Natural History Survey 31. 397 pp. [see p. 22]
Britton, W.E. 1923. Guide to the insects of Connecticut. Part IV. The Hemiptera or sucking insects of Connecticut. Bulletin. State of Connecticut. State Geological and Natural History Survey 34: 1-807. [See p. 24-55, Otiocerus on P. 41-42, also Van Duzee 1923b]
Brodie, W. & J.E. White. 1883. Check list of insects of the Dominion of Canada. C. Blackett Robinson, Toronto. 67 pp [65 in copy]. [see pp. 58-61]
Burmeister, H.C.C. 1835. Schnabelkerfe. Rhynchota. Handbuch der Entomologie 2(1): 1-396. [Planthoppers only, pp. 144-171] [Key, Anotia noted]
De Haas, M., B.W. Bahder & C.R. Bartlett. 2025. A new species of Anotia Westwood from Bonaire and Curaçao (Fulgoromorpha: Fulgoroidea: Derbidae: Otiocerini). Zootaxa 5627 (3): 539–550 https://doi.org/10.11646/zootaxa.5627.3.7
Dozier, H.L. 1928 [dated 1922 or 1926]. The Fulgoridae or planthoppers of Mississippi, including those of possible occurrence. Technical Bulletin of the Mississippi Agricultural Experiment Station 14: 1–1152. (Anotia p. 142)
Emeljanov, A.F. 1995. On the problem of a system and a phylogeny of the family Derbidae (Homoptera, Cicadina). Entomologicheskoe Obozrenie 73(4): 783–1811 & 946–1947. [Russian] [English Translation: Entomological Review 75(2): 70–1100]
Emeljanov, A.F. 1999. A new genus for Heronax candidus Anufriev and some taxonomic remarks concerning the Far East Derbidae (Homoptera, Cicadina). Zoosystematica Rossica 8(1): 120.
Fennah, R.G. 1952. On the generic classification of Derbidae (Fulgoroidea), with descriptions of new Neotropical species. Transactions of the Royal Entomological Society of London 103(4): 109–170.
Fitch, A. 1856. Third report on noxious and other insects of the State of New York. Transactions of the New-York State Agricultural Society 16: 315–490.
Fitch, A. 1893. Reprint of the Catalogue with references and descriptions of the insects collected and arranged for the State Cabinet of Natural History. Report of the New York State Museum of Natural History 46: 385-409. [Anotia p. 387]
Fowler, W.W. 1904. Order Rhynchota. Suborder Hemiptera-Homoptera. (Continued). Biologia Centrali-Americana 1: 57–76, 77–84. [P. 77]
Gonzon, A.T., Jr., C.R. Bartlett & J.L. Bowman. 2007 (dated 2006). Planthopper (Hemiptera: Fulgoroidea) diversity in the Great Smoky Mountains National Park. Transactions of the American Entomological Society 132: 243–260.
Kirby, W. 1821. The characters of Otiocerus and Anotia, two new genera of Hemipterous insects belonging to the family of Cicadiadae: with a description of several species. Transactions of the Linnean Society of London (Second series) Zoology 13: 12–123.
Leonard, M.D. 1928a. “Families Cercopidae, Membracidae, and Cicadellidae.” In: A list of the insects of New York with a list of the spiders and certain other allied groups. Memoir. Cornell University Agricultural Experiment Station 101: 1-1121. [PDF of Fulgoroidea only]
Maw, H.E.L., R.G. Foottit & K.G.A. Hamilton. 2000. Checklist of the Hemiptera of Canada and Alaska, NRC Research Press, Ottawa, Canada.
Metcalf, Z.P. 1913b. The wing venation of the Fulgoridae. Annals of the Entomological Society of America 6: 341-352.
Metcalf, Z.P. 1923. A key to the Fulgoridae of Eastern North America with descriptions of new species. Journal of the Elisha Mitchell Scientific Society 38(3): 139-230, plus 32 plates.
Metcalf, Z.P. 1938. The Fulgorina of Barro Colorado and other parts of Panama. Bulletin of the Museum of Comparative Zoology, Harvard Coll. 82: 277-423. [available from http://www.biodiversitylibrary.org]
Metcalf, Z.P. 1945. Fulgoroidea (Homoptera) of Kartabo, Bartica District, British Guiana. Zoologica [Scientific contributions of the New York Zoological Society] 30(3): 125-143. (see p. 129)
Metcalf, Z.P. 1945. General Catalogue of the Hemiptera. Fascicle IV, Fulgoroidea, Part 4, Derbidae. Smith College, Northhampton, Massachusetts. [see p. 144]
Moore, G.A. 1950a. Catalogus des hémiptères de la province de Québec. Le Naturaliste Canadien 77: 233-271.
Moore, G.A. 1950b. Check-list of Hemiptera of the province of Quebec. Contributions de l’Institut de Biologie de l’Université de Montréal. 26: 1-49.
Muir, F.A.G. 1917. New Hawaiian Delphacidae. Proceedings of the Hawaiian Entomological Society 3: 298-311.
Muir, F.A.G. 1918d. Notes on the Derbidae in the British Museum collection.-II. Derbidae. Entomologist’s Monthly Magazine 54: 228-243.
Nixon, P.L. & J. E. McPherson. 1977. An annotated list of the phytophagous insects collected on immature black walnut trees in southern Illinois. Great Lakes Entomologist 10: 211-222.
Oman, P. W. 1947. The types of Auchenorrhynchous Homoptera in the Iowa State College Collection. Iowa State College Journal of Science 21: 161–228.
Paiero, S.M., S.A. Marshall & K.G.A. Hamilton. 2003. New records of Hemiptera from Canada and Ontario. Journal of the Entomological Society of Ontario 134: 115–129.
Palmer, W.A. & K.R. Pullen. 2001 . The phytophagous arthropods associated with Senna obtusifolia (Caesalpiniaceae) in Mexico and Honduras and their prospects for utilization for biological control. Biological Control 20: 76-83. [Anotia listed]
Packard, A.S. 1888 (dated 1889). Hemiptera. Pp. 514–553. In: Guide to the study of insects: and a treatise on those injurious and beneficial to crops ; for the use of colleges, frm-schools, and agriculturists. 9th ed. Henry Hold and Company. New York. Pp.: i-xii, 1-715. Plate(s): 1-15 (see also p. 714).
Rathvon, S.S. 1869. Homoptera. Pp. 550–551. in: Mombert’s An authentic history of Lancaster County, in the State of Pennsylvania. Pp. viii + 617 (+ 175 pp appendix) [A list of species.]
Smith, J.B. 1890. Sub-order Homoptera. Pp. 436-447. In: Catalogue of insects found in New Jersey. Geological Survey of New Jersey. Final Report of the State Geologist, volume 2. John L. Murphy, Trenton, New Jersey 2 (2):1–486. [p. 439]
Smith, J.B. 1910. Order Homoptera. Pp. 87–130. In: A report of the insects of New Jersey. Annual Report of the New Jersey State Museum 1909:1–888. (PDF only to end of Auchenorrhyncha, p. 107; see link to BHL for whole volume) (see p. 94–98)
Spinola, M. 1839. Essai sur les Fulgorelles, sous-tribu de la tribu des Cicadaires, ordre des Rhyngotes. Annales de la Société Entomologique de France 8: 133–337.
Spooner, C.S. 1937. Derbid field days. Papers presented in the thirtieth annual meeting, Rockford, Illinois, May 7 and 8, 1937 (papers in Zoology). Transactions of the Illinois State Academy of Science 30(2): 315–316. (Euklastus reported among other derbid species)
Uhler, P.R. 1884. Hemiptera. Pp. 204-296. In: J. S. Kingsley. Standard natural history. Vol. II. Crustacea and Insects. S. E. Cassino and Co., Boston. Pp [VII] + 555 pp. [see p. 232]
Van Duzee, E.P. 1889. Observations on some northern Derbidae. (Conclusion). Canadian Entomologist 21: 176–179.
Van Duzee, E.P. 1893. New North American Homoptera. -N°.VI. Canadian Entomologist 25: 280-285.
Van Duzee, E.P. 1909. Synonymical notes on North American Homoptera. Canadian Entomologist 41(11): 380–384. (Anotia and Amalopota)
Van Duzee, E.P. 1916. Check list of Hemiptera (excepting the Aphididae, Aleurodidae and Coccidae) of America North of Mexico. New York Entomological Society, New York. 111 pp. [see p. 78 onward]
Van Duzee, E.P. 1917. Catalogue of the Hemiptera of America North of Mexico (excepting the Aphididae, Coccidae and Aleurodidae). University of California Publications, Technical Bulletins, vol. 2. University of California Press, Berkeley, pp. i-xiv, 1-902. [from Google books] [see p. 716 onward]
Westwood, J.O. 1840. The seventh order of insects, — the Hemiptera (Rhyngota, Fabr.). Pp. 562-573. In: Cuvier’s Animal Kingdom, arranged according to its organization; forming the basis for a natural history of animals, and an introduction to comparative anatomy. Wm. S. Orr and Co.. London. Pp.: i-vii, 1-670. Fig(s).: 1–300.
Wirtner, P.M. 1904. A preliminary list of the Hemiptera of western Pennsylvania. Annals of the Carnegie Museum. 3:183–232.
Wilson, S.W. & J.E. McPherson. 1980a. Keys to the planthoppers, or Fulgoroidea, of Illinois (Homoptera). Transactions of the Illinois Academy of Science 73(2): 1–61.
Wilson, S.W. & J.E. McPherson. 1980b. The distribution of the Fulgoroidea of the eastern United States (Homoptera). Transactions of the Illinois Academy of Science 73(4): 7–20.
Wilson, S.W. & J.E. McPherson. 1980c. A list of the Fulgoroidea (Homoptera) of southern Illinois. Great Lakes Entomologist 13(1): 25-30.
Wilson, S.W., J.L. Smith & P.D. Calvert. 1993. Planthoppers of a Missouri tallgrass prairie (Homoptera: Fulgoroidea). Journal of the Kansas Entomological Society 66(1): 75-80.
Wilson, S.W., C. Mitter, R.F. Denno & M. R. Wilson. 1994. Evolutionary patterns of host plant use by delphacid planthoppers and their relatives. In: R. F. Denno and T. J. Perfect, (eds.). Planthoppers: Their Ecology and Management. Chapman and Hall, New York. Pp. 7-45 & Appendix.