
Catonia nava (Say, 1830) (Achilidae, Plectoderini) (Photos by Kimberley Shropshire, University of Delaware)
Achilidae is a moderate-sized family of planthoppers consisting of approximately 160 genera and 511 species (Bourgoin 2018). Achilidae is of worldwide distribution, but is most diverse in the tropical and south temperate regions (Metcalf 1948). The higher taxonomy of Achilidae is currently problematic (I have summarized current status of the classification of the Achilidae here), but all North American achilids are all in the subfamily Achilinae (Metcalf 1948, Emeljanov 1993). Metcalf (1948) recognized 2 subfamilies in Achilidae, the Apatesoninae and the Achilinae. Apatesoninae consisted at that time of 2 genera and 5 species restricted to the Neotropics. Fennah (1950) revised the genera of Achilidae of the world, recognizing 7 tribes, but no explicit subfamilies. Emeljanov (1992, 1993) subsumed Achilixiidae into Achilidae, and recognized 3 subfamilies, 2 of which (Achilixiinae and Bebaiotinae) were subdivisions of Achilixiidae. All Achilidae proper were retained in Achilinae, which were divided into 3 supertribes and 12 tribes. Liang (2001) moved Achilixiidae to Cixiidae, but did not address the question of the higher taxonomy of Achilidae (Achilixiidae appear to be sister to Achilidae, Urban & Cryan 2007). North of Mexico, Achilinae consists of the tribes Achilini, Myconini, and Plectoderini (Fennah 1950, O’Brien 1971, Emeljanov 1993).
Achilidae is represented north of Mexico by 55 species in 8 genera. Among the North American taxa, 6 of 8 genera are in the tribe Plectoderini, which were revised north of Mexico by O’Brien (1971). One species, Uniptera ampliata, is in Achilini. The remaining genus, Cixidia is in Myconini and can be identified to species using Beirne (1950) (as Epiptera) for the Canadian fauna, which includes 11 of the 14 species north of Mexico. Epiptera was made a subgenus of Cixidia by Anufriev (1969). California includes a disproportionately high percentage of the U.S. fauna with 28 species in 5 genera, of which 1 genus (Uniptera) and 13 species are endemic to the state. The genera Momar, Juniperthia, Uniptera and Xerbus are found only in the west, while Opsiplanon is only in the southeast. Catonia, Cixidia and Synecdoche are generally distributed. Achilids are generally more abundant in the southern U.S., although many species are widespread, particularly in the east.
Achilidae is most easily recognized as being dorsoventrally flattened with the forewings apically overlapping when at rest. When the wings are outstretched, the trailing margins of the forewings are concave. Uniptera ampliata departs somewhat from this general appearance. The second hind tarsomere bears a row of spines, a feature shared with Cixiidae and Kinnaridae, which are the most similar families. Of achilid genera found north of Mexico, Catonia is the most rich with 41 species, most of them Neotropical. Cixidia includes 40 species, all but 14 of these Palearctic. All species of 4 genera (Juniperthia, Synecdoche, Uniptera and Xerbus) occur north of Mexico, except a species of Synecdoche described from Vietnam (Fennah 1978). Opsiplanon (3 species) and Momar (3 species) include species from Central America or the Caribbean in addition to those north of Mexico.
Immatures are fungus feeders and are particularly found under the bark of dead logs (O’Brien 1971). Immatures are coated with a waxy material that apparently serves as protection from predators (Hepburn 1967, Liang & O’Brien 2002). Cixidia are evidently associated with pines (Hepburn 1967, Wilson 1983). Adults feed on woody plants, and are more often associated with gymnosperms than other planthopper families (Wilson et al. 1994). Achilids have not been reported as economic pests (Wilson & O’Brien 1987). The degree of host specificity of adults is not clear (O’Brien 1971), although species are most often reported as polyphagous (Wilson et al. 1994). Achilids may exhibit a single generation per year (Bartlett et al. 2011), but adults are not closely synchronous (O’Brien 1971). Achilids are most readily collected at lights.
The classification of the Achilidae north of Mexico can be summarized as follows:
Family Achilidae
Achilinae Stål, 1866
Achilini Stål, 1866
= Elidipterini Fennah 1950; syn. by Emeljanov 1992: 53.
Uniptera Ball, 1933 (Type species Uniptera ampliata Ball, 1933a).
Myconini Fennah, 1950
Cixidia Fieber, 1866a (Type species Cicada confinis Zetterstedt, 1828).
= Epiptera Metcalf, 1922; status (subgenus) by Anufriev 1969: 173-174 (Type species Flata opaca Say, 1830).
Plectoderini Fennah, 1950
Catonia Uhler, 1895 (Type species Flata nava Say 1830).
Juniperthia O’Brien, 1985 (Type species Catonia succinea Van Duzee, 1916b); replacement name for preoccupied Juniperia O’Brien, 1971 (nec. Linnavuori 1965).
= Juniperia O’Brien, 1971 (nec. Linnavuori 1965); syn. by O’Brien 1985.
Momar Fennah, 1950 (Type species Plectoderes lineatocollis Fowler, 1904).
Opsiplanon Fennah, 1945 (Type species Opsiplanon ornatifrons Fennah, 1945).
Synecdoche O’Brien, 1971 (Type species Catonia grisea Van Duzee, 1908).
Xerbus O’Brien, 1971 (Type species Catonia brunella Ball, 1933).
Identification of achilid genera north of Mexico (modified from O’Brien, 1971).
1. Costal cell broad, at its widest point 1/3 as wide as forewing; wings rather sinuate along costal margin; California … Uniptera Ball
1’. Costal cell narrow, never more than ¼ as wide as forewing; wings not sinuate along costal margin … 2
2. Head, including eyes, less than 2/3 as wide as pronotum … Cixidia Fieber
2’. Head including eyes at least 2/3 as wide as pronotum … 3
3. Hind tibiae with spine in basal half, medioventral lobe of male pygofer present; frons less than 1.5x as long medially as broad … 4
3’. Hind tibiae without spine in basal half; medioventral lobe of male pygofer reduced or absent; frons 1.5-2x (usually 2x) as long as broad; California, southwestern … Juniperthia O’Brien
4. Sc+R fork of forewing near level of union of claval veins, subcostal cell about 1/3 length of forewing or longer; medioventral lobe of male pygofer entire or bifurcate … 5
4’. Sc+R fork of forewing near stigma, subcostal cell 1/6 length of forewing, widest medially; medioventral lobe of male pygofer trilobed at apex … Opsiplanon Fennah
5. Subcostal cell of forewing longer than 1/3 length of wing, narrow throughout; medioventral lobe of male pygofer entire … 6
5’. Subcostal cell of forewing about 1/3 length of wing, wider before its apex; medioventral lobe of male pygofer bifurcate … Catonia Uhler
6. Rostrum longer than clypeus, reaching base of hind coxae; pronotum medially shorter than tegulae, or if not, then mesonotum with lateral carinae straight … 7
6.’ Rostrum as long as clypeus; pronotum medially longer than tegulae, and mesonotum with lateral carinae bent or rounded; southwestern … Xerbus O’Brien
7. Lateral lobe of phallobase broadened dorsoventrally, dorsal lobe reduced; frons with three pairs of dark spots, sometimes fused, on basal half; southwestern … Momar Fennah
7’. Lateral lobe of phallobase not as above, dorsal lobe present; frons not so marked; widespread … Synecdoche O’Brien
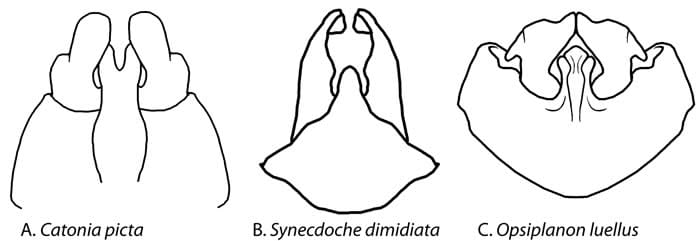
Male pygofer of achilid genera, ventral view (Drawings by Kimberley Shropshire, University of Delaware).
References
Anufriev, G. A. 1969. Studies on some Palearctic Achilidae (Homoptera, Auchenorrhyncha). Bulletin de l’Académie Polonaise des Sciences Serie des Sciences Biologiques 17(3): 173-178.
Ball, E. D. 1933. Some new western leafhoppers of the Fulgorid family Achilidae. Psyche 9: 133-138.
Bartlett, C. R., E. R. Adams and A. T. Gonzon. 2011. Planthoppers of Delaware (Hemiptera, Fulgoroidea), excluding Delphacidae, with species incidence from adjacent States. ZooKeys 83: 1-42.
Bartlett, C. R., L. B. O’Brien and S. W. Wilson. 2014. A review of the planthoppers (Hemiptera: Fulgoroidea) of the United States. Memoirs of the American Entomological Society 50: 1-287.
Beirne, B. P. 1950. The Canadian species of Epiptera (Homoptera: Achilidae). Canadian Entomologist 82: 186-190.
Bourgoin, T. 2018. FLOW (Fulgoromorpha Lists on The Web): a world knowledge base dedicated to Fulgoromorpha. Version 8, updated 26 May 2018, accessed July 5, 2018. Available http://flow.snv.jussieu.fr/.
Emeljanov, A. F. 1992. Toward the problem and limits and subdivisions of Achilidae (Homoptera, Cicadina). Entomological Review 71(1): 53-73 (Translation of Entomologicheskoye Obozreniye 1991, 70: 373-393, in Russian).
Emeljanov, A. F. 1993. Description of tribes of the subfamily Achilinae (Homoptera: Achilidae) and revision of their composition. Entomological Review 72(6): 7-27.
Fennah, R. G. 1950. A generic revision of the Achilidae (Homoptera: Fulgoroidea) with descriptions of new species. Bulletin of the British Museum (Natural History) Entomology 1: 1-170.
Fennah, R. G. 1978. Fulgoroidea (Homoptera) from Vietnam. Annales Zoologici 34(9): 207-279.
Hepburn, H. R. 1967. Notes on the genus Epiptera (Homoptera: Achilidae). Journal of the Georgia Entomological Society 2(3): 78-80.
Liang, A. P. 2001. Morphology of antennal sensillae in Achilixius sandakanensis Muir (Hemiptera: Fulgoromorpha: Achilixiidae) with comments on the phylogenetic position of the Achilixiidae. The Raffles Bulletin of Zoology 49: 221-225.
Liang, A. P. and L. B. O’Brien. 2002. External morphology of the wax glands of Epiptera woodworthi (Hemiptera: Fulgoromorpha: Achilidae). Southwestern Entomologist 27(2): 209-215.
Metcalf, Z. P. 1948. General Catalogue of the Hemiptera. Fascicle IV, Fulgoroidea, Part 10 Achilidae. Smith College, Northhampton, Massachusetts.
O’Brien, L. B. 1971. Systematics of the tribe Plectoderini (Insecta, Fulgoroidea, Achilidae) in America North of Mexico. University of California Publications in Entomology 64: 1-79.
Urban, J. M. and J. R. Cryan. 2007. Evolution of the planthoppers (Insecta: Hemiptera: Fulgoroidea). Molecular Phylogenetics and Evolution 42: 556-772.
Wilson, S. W. 1983. Description of the fifth instar of Epiptera opaca (Homoptera: Fulgoroidea: Achilidae). Great Lakes Entomologist 16(1): 1-3.
Wilson, S. W., C. Mitter, R. F. Denno and M. R. Wilson.1994. Evolutionary patterns of host plant use by delphacid planthoppers and their relatives. In: R. F. Denno and T. J. Perfect, (eds.). Planthoppers: Their Ecology and Management. Chapman and Hall, New York. Pp. 7-45 & Appendix







