Jianyu Xu and Cameron Twitty’s recent publication, “Nickel-Catalyzed Deaminative Cyanation: Nitriles and One-Carbon Homologation from Alkyl Amines“, is highlighted in Synform (link)! Thank you to Dr. Matteo Zanda for including our work!
From Pyridiniums to Chiral Amines
Congratulations to Kristen Baker, Amanda Tallon (Fox Research Group), Olivia Bercher, and our Pfizer collaborators Matt Perry and Richard Loach on their method to prepare α-chiral amines via the reaction of alkylpyridinium salts and sulfinimines (link)! It’s our first foray into non-nickel catalyzed reactions of alkylpyridinium salts, and proceeds via a rare thermally promoted SET of an anion–π complex of the pyridinium and carbonate anion.

Converting NH2 to CH3
Congratulations to Olivia Bercher, Shane Plunkett, and Tom Mortimer for their development of a nickel-catalyzed reductive methylation of alkylpyridinium salts (link)! This method enables amino groups to be converted into methyls via pyridinium salt intermediates.

New Route to Alkyl Nitriles
Congratulations to Jianyu Xu and Cameron Twitty on their report of a nickel-catalyzed cya nation of alkylpyridinium salts (link)! Now chemists can transform alkyl amines to nitriles and perform one-carbon homologations without complete resynthesis.

Congratulations, Dr. Kristen Baker!
Congratulations, Dr. Kristen Baker, on defending your thesis, “Deaminative Couplings of Alkylpyridinium Salts!” Great research and great story. It was also super special to celebrate in person! We miss you already and wish you all the best in your postdoc at Providence College!!

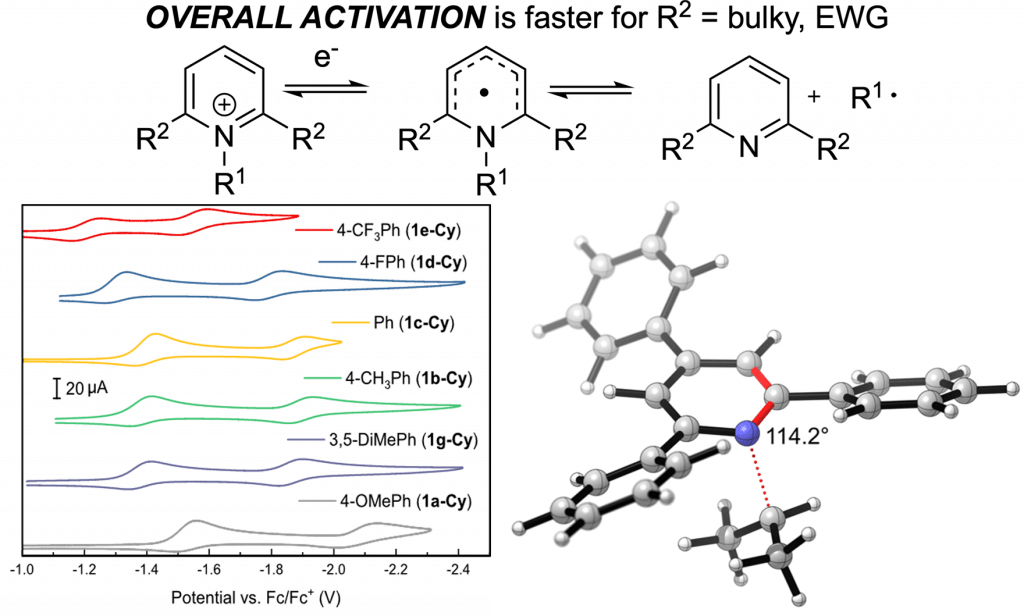
Understanding How Pyridinium Structure Influences Deamination
In this collaborative effort with the Kozlowski and Rosenthal groups, we investigate the effect of pyridinium substituents on the efficiency of reduction and C–N bond cleavage. You may have wondered if those 2,6-aryl groups were really needed… This paper answers that question and then delves deeper into the electronic and steric factors that promote deamination. We learned so much in this collaboration and are excited to see it published in ACS Catalysis.
New Method for Stereospecific Quat Center Formation
We’re so excited to see Jianyu and Olivia’s work published (link)! Jianyu discovered that the addition of a stilbene enables stereospecific cross-couplings of benzylic carboxylates without naphthyl substitution! Olivia’s mechanistic studies show that there’s more to learn about this intriguing catalyst system (more to come on that). Congrats to Jianyu and Olivia!
New Artwork
Thanks to the fabulous Samantha Santana for our new group portrait! We love this new header image (and the way you captured our personalities)!!
Our Perspective on Stereospecific Cross-Couplings
Jianyu Xu, Olivia Bercher, and Mike Talley collaborated to provide our perspective on nickel-catalyzed, stereospecific C–C and C–B cross-couplings using substrates derived from amines and alcohols (link). Great job, Jianyu, Olivia, and Mike!
Enantioselective Alkynylation of Unstabilized Cyclic Iminium Ions
Weiye Guan, Samantha Santana, Jennie Liao, and Kelci Henninger have developed a highly enantioselective, copper-catalyzed alkynylation of unstabilized cyclic iminium ions (link). This reaction delivers alpha-chiral amine heterocyclic with easily deprotected nitrogen protecting groups. The key was squeezing the chiral pocket by adding ethyl groups to the PyBox ligand. Congratulations to the team for this great method!
Also unveiling Sam’s awesome artwork celebrating Weiye’s discoveries and PhD!

Congratulations, Dr. Jianyu Xu!
Congratulations, Dr. Weiye Guan!
Mitchell Daneker is a ACS DOC SURF Fellow!
 We are thrilled that Mitchell Daneker was selected for a fellowship from the American Chemical Society Division of Organic Chemistry Summer Undergraduate Research Fellowship program! Congratulations to Mitchell and thank you to the ACS DOC!
We are thrilled that Mitchell Daneker was selected for a fellowship from the American Chemical Society Division of Organic Chemistry Summer Undergraduate Research Fellowship program! Congratulations to Mitchell and thank you to the ACS DOC!
Congratulations, Dr. Megan!
Congratulations, Dr. Megan Hoerrner, on defending your thesis “Nickel-Catalyzed Cross-Coupling Reactions Through C–O and C–N Bond Activation.” This is the 11th PhD defense from the group, and our first one via Zoom videoconference! Best wishes for your next position at Vertex Pharmaceuticals! We miss you!!

Congratulations, Dr. Javon!
Our Latest Pyridinium Papers
Congratulations to Megan Hoerrner, Kristen Baker, Corey Basch, and Earl Bampo for their Deaminative Arylation of Amino Acid-derived Pyridinium Salts! This method enables amino acids to be converted to propionic acid derivatives.
Congrats also to Kristen Baker, Diana Lucas Baca, Shane Plunkett, and Mitchell Daneker for their alkyl–alkyl cross-coupling of pyridinium salts with alkyl boranes generated in situ from alkene hydroboration! We submitted this one on Halloween, so Mitchell celebrated with a hot chocolate and dressing as a Dixie cup (see photo).
Alkyl–Alkyl Cross-Coupling Highlighted in Synfacts
Shane, Corey, and Samantha’s alkyl–alkyl cross-coupling of alkyl pyridinium salts was highlighted in Synfacts. Thank you to Prof. Paul Knochel and Ferdinand H. Lutter for describing our work!
Reductive Couplings of Alkylpyridinium Salts
In collaboration with Michelle Garnsey, Brian Boscoe, and Joe Tucker at Pfizer, Inc., Jennie Liao, Corey Basch, Megan Hoerrner, and Mike Talley developed a reductive cross-electrophile coupling of alkylpyridinium salts with aryl bromides (link). Congratulations to the team for their great work! Congrats also to Prof. Ruben Martin’s team, who developed a similar reaction (link)!
Alkyl–Alkyl Cross-Coupling Highlighted in OPRD
Group members Shane Plunkett, Corey Basch, and Samantha Santana have developed a Negishi alkylation of alkylpyridinium salts. This is the first example of alkyl–alkyl cross-coupling of alkylpyridinium salts with unactivated alkyl groups. Read it all in their J. Am. Chem. Soc. paper (link). This work was highlighted in Org. Process Res. Dev. Congratulations to this team for a great new reaction!