In addition to adaptation and co-contraction, online feedback responses are essential mechanisms of motor control. Online responses occur at multiple time scales, ranging from short latency spinal responses (less than 50 ms in the upper extremity) to voluntary responses (more than 100 ms). In between these responses, the long-latency responses are interesting because they blend fast reaction times with flexibility of their output, but their neural correlates are not yet completely understood. We call this line of research StretchfMRI, because we aim to combine MR-compatible robotics, fMRI, and EMG to study the neural correlates of long-latency responses. Utilizing surface EMG activity, we measure the LLR amplitude evoked from flexor and extensor muscles following the application of controlled angular displacements of the wrist using the MR-StretchWrist.
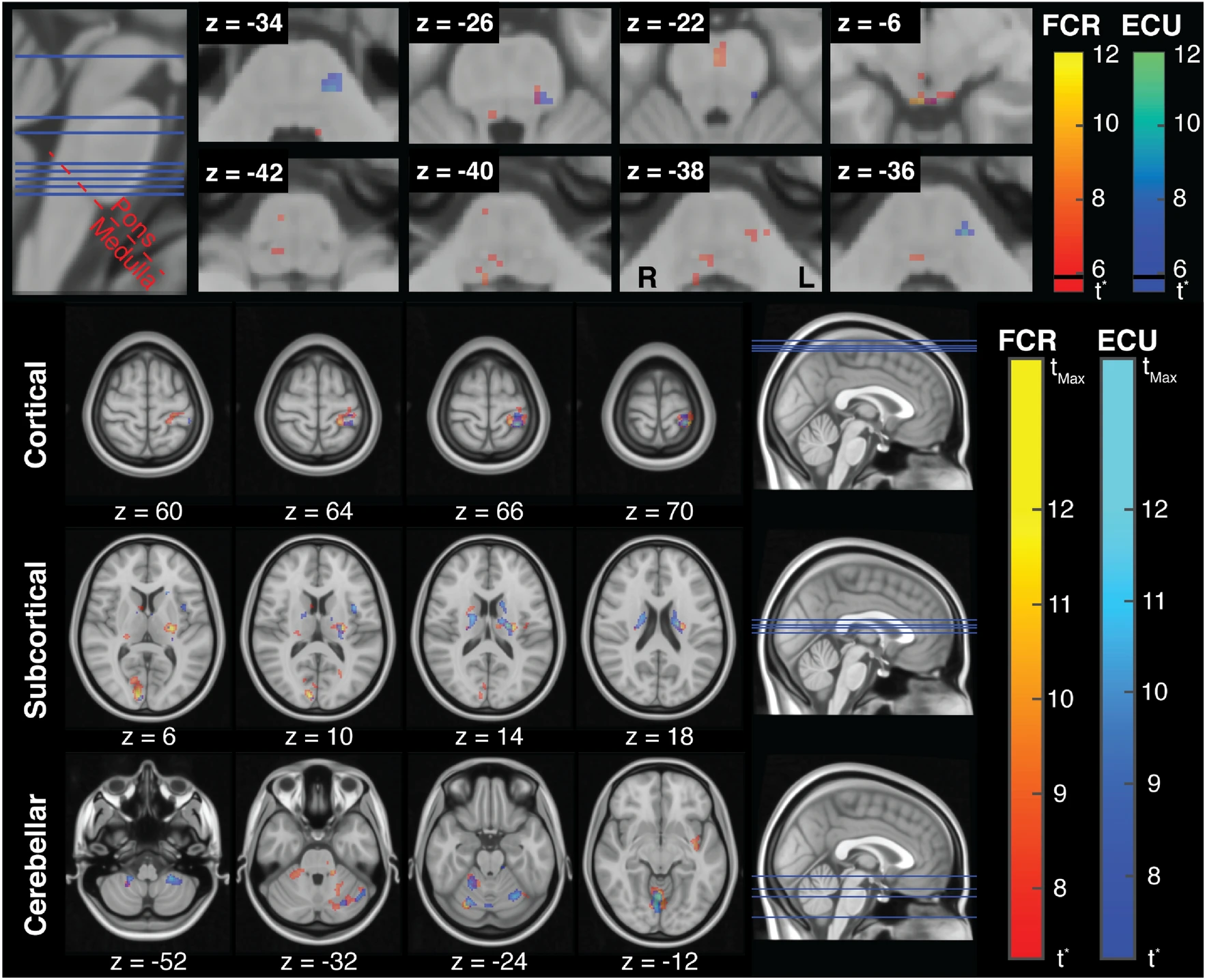
Our earliest work on this topic was on developing StretchfMRI, a measurement method that enables simultaneous measurement of fMRI and EMG signals during stretch-evoked muscle responses (see paper by Zonnino below). Our methods allowed us to establish for the first-time in human the organization of LLRs in the brainstem using fMRI.
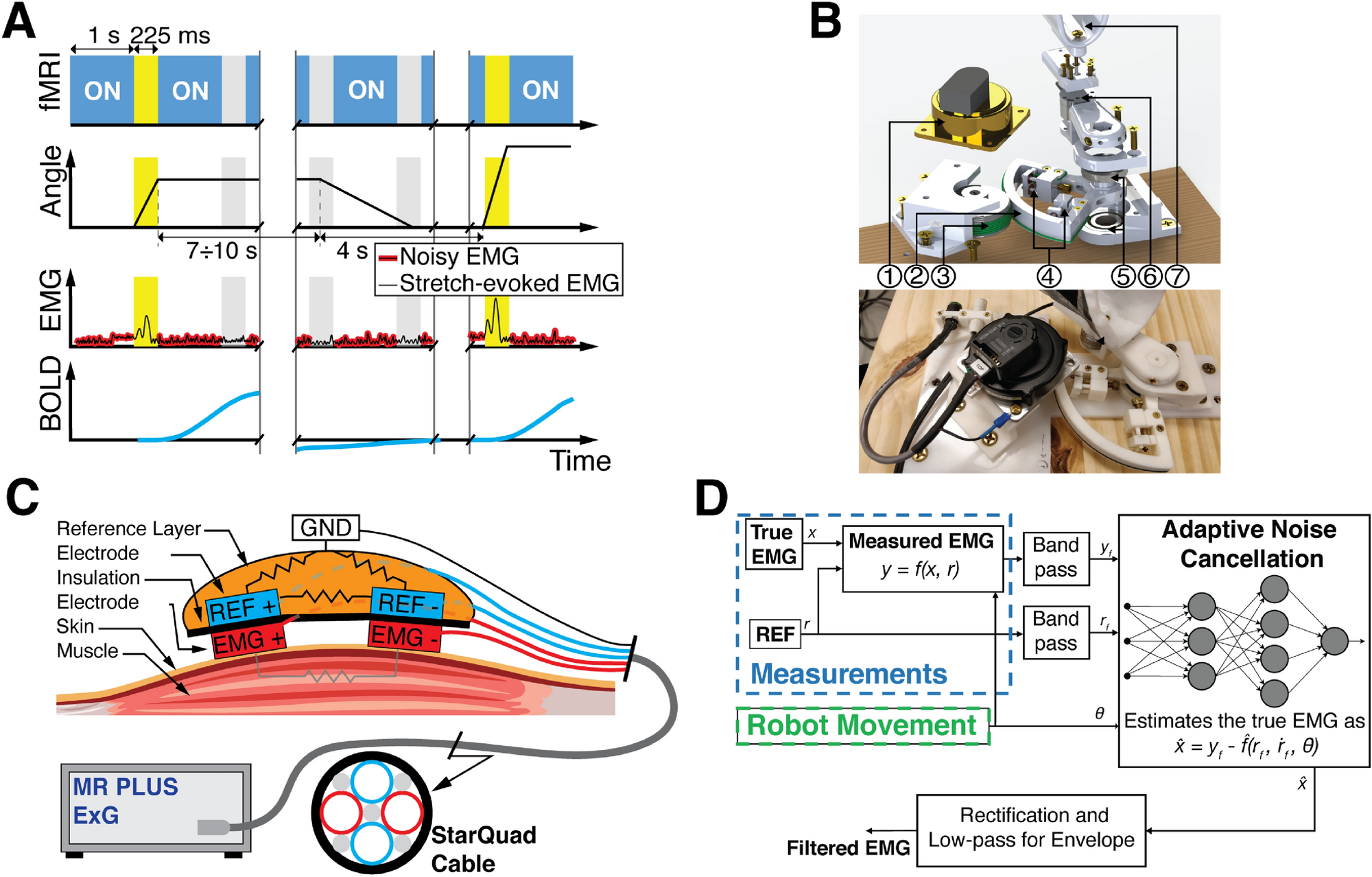
(above) Timing diagram, MR-StretchWrist robot design, and EMG acquisition for the StretchfMRI protocol.
(above) Muscle specific organization of stretch-related activity in the brainstem. Stretch-related activity in the cortical, subcortical, and cerebellar brain regions associated with FCR and ECU activity.
In parallel to our methods development work, we have conducted a study to quantify how different behavioral factors including the neuromechanical state of the muscle prior to the application of a perturbation (i.e., muscle length and activation), the direction of perturbation (i.e., whether the perturbation stretches or shortens the muscle), the kinematic features of the applied perturbation (i.e., perturbation velocity, duration, amplitude, velocity profile), and the instructions provided to participants as to how to respond to the applied perturbations may affect the long-latency responses (LLR) amplitude evoked from the forearm muscles.
Our current work on this topic includes the development of a higher-power robot (the Dual Motor StretchWrist) to study the responses under a “yield” and “resist” condition. We have validated the performance of the Dual Motor Stretch-Wrist (DMSW) to provide consistently and precisely timed wrist perturbations in both the flexion and extension directions. We collected behavioral data to determine the effect of task instruction (yield or resist) on the long-latency response and wrist torque following perturbation onset using the DMSW in a mock-scanner setup outside of the MRI. We collected preliminary fMRI data validating the MR-compatible of the DM-SW and its capability in eliciting stretch reflex responses when participants are resisting while inside the MRI scanner.
We are currently working on combining TMS with our StretchfMRI paradigm, which would allow to decouple the effects of corticospinal and reticulospinal circuits on the muscle and neural responses observable via StretchfMRI. We quantified the effect of subthreshold TMS applied to the motor cortex to the long-latency response amplitude in a forearm flexor muscle. This work demonstrates the capability of subthreshold TMS to significantly reduce the long-latency response amplitude in a forearm flexor muscle and highlights the specific subthreshold TMS intensity and timing needed to achieve such a reduction. We are in the process of combining TMS with StretchfMRI allowing to quantify the neural correlates and muscle activity associated with wrist perturbations in presence of subthreshold TMS. This paradigm enables us the capability of applying single TMS pulses to the motor cortex during functional MRI with surface EMG measurements during the wrist perturbations inside the scanner.

(above) Physical setup of the simultaneous TMS-fMRI experimental paradigm on the MRI scanner bed.
Publications on this topic
C. A. Helm, F. Sergi, “Inhibitory Effect of Subthreshold TMS on the Long-Latency Response in the Flexor Carpi Radialis”, 2024, doi: 10.1101/2024.03.18.585555, pre-print.
R. C. Nikonowicz, F. Sergi, “Development of an MRI-compatible robotic perturbation system for studying the task-dependent contribution of the brainstem to long-latency responses”, 2024, doi: 10.1101/2024.03.01.583025, pre-print.
A. Zonnino, A. J. Farrens, D. Ress, F. Sergi, “Measurement of stretch-evoked brainstem function using fMRI”, Scientific reports, vol. 11, no. 12544, 2021, pre-print, available online.
J. Weinman, P. Arfa-Fatollahkhani, A. Zonnino, R. C. Nikonowicz, F. Sergi, “Effects of Perturbation Velocity, Direction, Background Muscle Activation, and Task Instruction on Long-Latency Responses Measured From Forearm Muscles”, Frontiers in Human Neuroscience, vol. 15, 2021, pre-print, available online.
A. Zonnino, A. J. Farrens, D. Ress, F. Sergi, “StretchfMRI: a novel technique to quantify the contribution of the reticular formation to long-latency responses via fMRI”, 16th International Conference on Rehabilitation Robotics, 2019, pre-print, available online.