[back to Higher Classification of Delphacidae]
[back to A checklist of New World delphacid species]
Contents
Family Delphacidae Leach, 1815
Subfamily Delphacinae Leach, 1815
Tribe Tropidocephalini Muir, 1915
Genus Lamaxa Bartlett & Kennedy, 2018 (abstract)
Type species (in original combination): Malaxa occidentalis Muir, 1926.
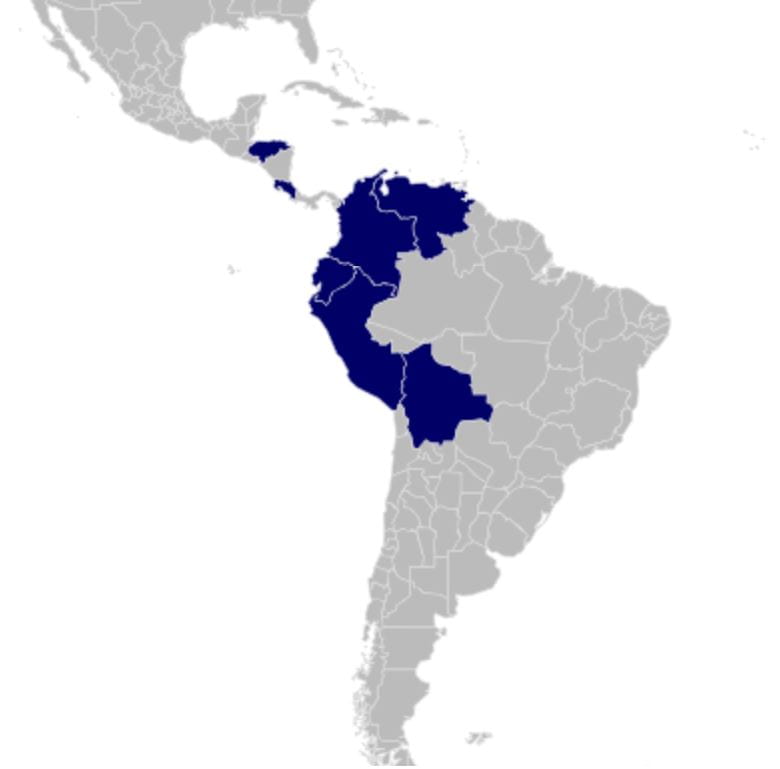
Distribution
Neotropical – southern Mesoamerica and Northern South America.
Recognized species
There are 2 species placed in this genus, removed from Malaxa by Bartlett & Kennedy (2018).
Lamaxa gracilis (Fennah, 1945: 430) – Venezuela
= Malaxa gracilis Fennah, 1945: 430.
= Lamaxa gracilis (Fennah, 1945); new comb. by Bartlett & Kennedy, 2018: 519.
Lamaxa occidentalis (Muir, 1926: 7) – Bolivia, Ecuador, Colombia, Costa Rica; tentatively also Peru
= Malaxa occidentalis Muir, 1926: 7
= Lamaxa occidentalis (Muir, 1926); new comb. by Bartlett & Kennedy, 2018: 517.
Plant associations
Lamaxa occidentalis (Muir, 1926) – Gynerium sp. (Poaceae: Panicoideae: Gynerieae)
Gynerium sp. (caña brava, giant reed, or wildcane), as currently understood, consists of a single species, Gynerium sagittatum (Aublet) P. Beauvois, distributed from the Caribbean and southern Mexico to tropical Argentina (Sánchez-Ken & Clark 2001). The species is a reed-like grass, and among the tallest grasses, excluding woody bamboos, reaching 10-15 m in height (Sánchez-Ken & Clark 2001), and is a pioneer species of floodplain forests along whitewater rivers with rapid channel migration rates (Kalliola et al. 1992). While Gynerium is not phylogenetically close to the bamboos (e.g., Soreng et al. 2015), it is physiognomically similar, and if Gynerium is a correctly reported plant association, Lamaxa and other Tropidocephalini reported to be associated with Gynerium, may be more widely distributed than currently understood.
Names of plants from USDA PLANTS database, host data from Muir (1926).
Economic Importance
Not reported as pests.
Recognition
Key to Genera of New World Tropidocephalini
(revised from Bartlett and Kennedy 2018).
1. Body strongly dorsoventrally flattened; frons rather square, median carinae of frons forked ventrally near lower margin of eyes … Procidelphax Bartlett
– Body not flattened, frons rectangular, median carinae not forked, except dorsally near fastigium in some species … 2
2. Vertex much (ca. 1.5x) longer than broad, rounded anteriorly in dorsal view; median carina of vertex unbranched … Macrocorupha Muir
– Vertex shorter, more truncate anteriorly; median carina of vertex variable … 3
3. Antennae very long, exceeding posterior margin of mesothorax; both segments long (much longer than wide) with segment I nearly ½ length segment II … Lamaxa Bartlett & Kennedy.
– Antennae not as long, not exceeding mesothorax; generally segment I somewhat longer than wide and 1/3 or less length of II … 4
4. Lateral carinae of pronotum reaching hind margin; the Y-carina of vertex not distinct, sometimes forming a rounded areolet at apex of the median carina; anal tube with or without processes (a heterogeneous taxon, often strongly marked on body and wings) Columbisoga Muir
– Lateral carinae of pronotum not attaining hind margin; the Y-carinae of vertex distinct, rounded areolet not present; anal tube without processes … 5
5. Wings clear and unmarked (except fuscous at wing base in C. caresi); head and body uniformly colored; ventral margin of pygofer with broad forked process (also with a pair of lateral projections in the type species C. lloydi); aedeagus caudally directed or somewhat twisted, not strongly curved ventrad … Columbiana Muir
– Wings clear, strongly marked with fuscous; body dark, marked with pale (especially on head); ventral margin of pygofer with broad scoop-like projection (not apically forked) and a pair of lateral teeth; aedeagus strongly downcurved … Xalama Bartlett & Kennedy
Description – (abridged and slightly modified from Bartlett & Kennedy 2018)
Color. Brownish-orange with brown and paler markings; wings clear with characteristic dark (and sometimes pale) markings. Structure. Body slender and elongate; length (from apex of vertex to tip of tegmina) males 4.71–5.33 mm; females 5.10–5.62 mm. Head much narrower than pronotum, carinae concolorous. Lateral carinae of clypeus, frons (~metope) and vertex (~coryphe) distinct; other carinae obscure, especially near the fastigium. Frons elongate and parallel-sided, widest near frontoclypeal suture, narrowed to fastigium (l:w = 2.6:1). Vertex longer than broad at base (l:w = 1.4:1), distally narrowing and projected slightly in front of eyes; submedian carinae uniting before fastigium, basal compartments longer than wide. Rostrum reaching or exceeding metathoracic trochanters. Antennae cylindrical, both segments very long, surpassing apex of clypeus (and apex of mesonotum), segment I close to half length of II (ratio I:II 0.4–0.5:1), length antennal segment I =0.47 mm, II = 1.05 mm. Pronotum shorter than vertex in middle line, lateral carinae usually not attaining hind margin. Mesonotum longer in middle line than vertex and pronotum together. Wings elongate, much longer than abdomen, nodus at about 2/3 length, apex acutely rounded. Spinal formula of hind leg 5-7-5 (or 5-6-5). Calcar thick, concave on inner surface, without teeth along the hind margin, with an apical tooth. Pygofer irregularly quadrilateral in lateral view. In caudal view, opening carinate, with midventral forked process, broad lamellate medioventral processes absent. Dorsocaudal margins of pygofer expanded and inflected to partially enclose anal tube, diaphragm evident, armature absent. Gonostyli broad basally, basal angles prominent, diverging and narrowed distally to apically to blunt or acute apex. Aedeagus elongate and tubular with large, flattened, poorly sclerotized subapical process (representing a flagellum?). Anal tube (anal segment) small, without processes.
Lamaxa occidentalis
Online Resources
iNaturalist.
TaxonPages.
EOL.
FLOW
Plazi
GBIF
Molecular resources
There are no data on this genus in Genbank or BOLD.
Selected references
Bartlett, C.R. 2010 (dated 2009). A new genus of new world Tropidocephalini (Hemiptera: Delphacidae: Delphacinae), with the description of two new species. Entomological News 120(4): 387-396.
Bartlett, C.R. & A.C. Kennedy. 2018. A review of New World Malaxa (Hemiptera: Fulgoroidea: Delphacidae). Zootaxa 441(3): 511-528. http://dx.doi.org/10.11646/zootaxa.4441.3.5.
Fennah, R.G. 1945. The Fulgoroidea, or lanternflies, of Trinidad and adjacent parts of South America. Proceedings of the United States National Museum 95(3184): 411-520.
Kalliola, R., M. Puhakka & J. Salo. 1992. Intraspecific variation, and the distribution and ecology of Gynerium sagittatum (Poaceae) in the western Amazon. Flora, 186 (3–4), 153–167. https://doi.org/10.1016/S0367-2530(17)30531-5.
Leach, W.E. 1815. Entomology. The Edinburg encyclopedia; conducted by David Brewster 9: 57-172. (family Delphacidae p. 125).
Metcalf, Z.P. 1943. General Catalogue of the Hemiptera. Fascicle IV, Fulgoroidea, Part 3, Araeopidae (Delphacidae). Smith College, Northhampton, Massachusetts. 552 pp.
Muir, F.A.G. 1926. Contributions to our knowledge of South American Fulgoroidea (Homoptera). Part I. The Family Delphacidae. Experiment Station of the Hawaiian Sugar Planters’ Association, Entomological Series, Bulletin 18:1-51, plates 1-5.
Muir, F.A.G. 1930. On some South American Delphacidae (Homoptera, Fulgoroidea). Entomologisk Tidskrift 51(3-4): 207-215.
Qin Dao-Zheng, Ya-Lin Zhang & Jin-Hua Ding. 2006. A taxonomic study of the genus Bambusiphaga (Hemiptera, Fulgoroidea, Delphacidae). Acta Zootaxonomica Sinica 31(1): 148-151.
Sánchez-Ken, J., & L. Clark. 2001. Gynerieae, a New Neotropical tribe of grasses (Poaceae). Novon, 11 (3), 350–352. doi: 10.2307/3393044.
Soreng, R.J., P.M. Peterson, K. Romaschenko, G. Davidse, F.O. Zuloaga, E. J. Judziewicz, T.S. Filgueiras, J.I. Davis & O. Morrone. 2015. A worldwide phylogenetic classification of the Poaceae (Gramineae). Journal of Systematics and Evolution, 53(2), 117–137. doi:10.1111/jse.12150.