Fitchiella robertsoni (Fitch, 1856) (Caliscelidae) photo by Kimberley Shropshire (University of Delaware)
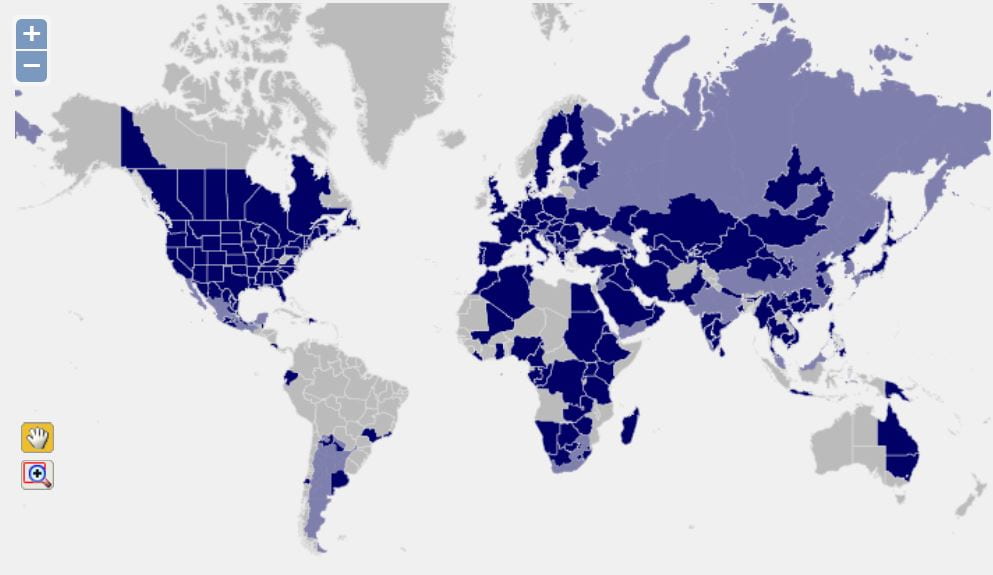
Caliscelidae is a small planthopper family, consisting of approximately 80 genera and 253 species (Bourgoin 2025). Caliscelids were elevated from a subfamily of Issidae to a family by Emeljanov (1999). Subsequently, an expanded family diagnosis and elaboration of tribal features were provided by Gnezdilov and Wilson (2006). Phylogenetic analyses support Caliscelidae as an independent family (e.g., Yang & Chang 2000, Yeh et al. 2005, Urban & Cryan 2007, etc.) allied with Eurybrachidae + Lophopidae, and the premise of Caliscelidae as a family is no longer seriously questioned.
Caliscelids are small, cryptic in habits, easily mistaken for nymphs (or beetles), and the most commonly encountered genera (especially Bruchomorpha) are diverse, leading caliscelids to be under-collected, under-reported, and poorly represented in museum collections. Species recognition is sometimes challenging. Caliscelids are distinctive among the North American fauna by being small, cylindrical planthoppers that are brachypterous (rarely macropterous), with the wings much shorter than the abdomen. Among the North American Issidae and Elicini (Tropiduchidae), only Osbornia may also have wings much shorter than the abdomen, but it has partially reticulated wings. Two genera of Caliscelidae have greatly expanded tibiae (front only in Caliscelis, front and middle in Fitchiella), and two genera (Fitchiella and Bruchomorpha) have the head projected similar to a weevil. Some caliscelids (e.g., Asarcopus, some Aphelonema) possess sexual dimorphism, but in Caliscelis, sexual dimorphism is very striking. Caliscelids have a single lateral spine on the hind tibiae, whereas Issidae and Elicini usually have more. Other morphological features of caliscelids overlap to some degree with Issidae and Elicini.
Caliscelidae is separated into 2 subfamilies, Caliscelinae with 2 tribes (Caliscelini and Peltonotellini) and Ommatidiotinae with 3 tribes (Ommatidiotini, Augilini, and Adenissini) (Gnezdilov and Wilson 2006, Gnezdilov 2011, 2014). Until recently, most Nearctic taxa were in Caliscelini (Caliscelinae), except the adventive Asarcopus palmarum (the date bug) and Papagona in Ommatidiotini (Ommatidiotinae); but subsequently, all New World Caliscelidae (except the adventive Caliscelis and Asarcopus ) were moved to Peltonotellini (e.g., Emeljanov 2008, Gnezdilov 2014).
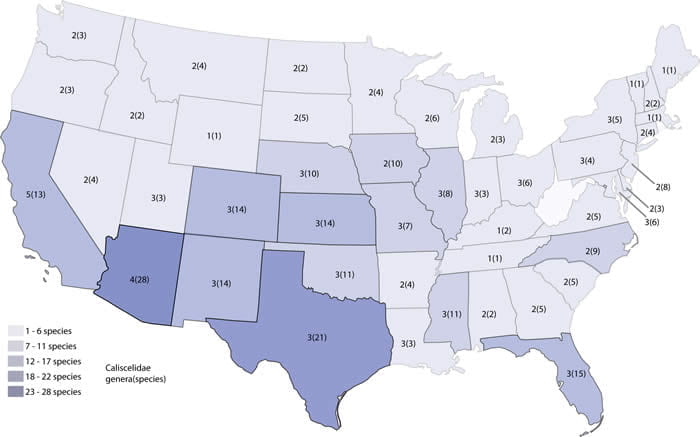
Caliscelidae north of Mexico include 6 genera and 53 species, with most of these in the central and southwestern states. Caliscelidae is species-rich throughout most of the United States, with the highest richness in the southwest and Great Plains. Three genera (Papagona and the adventive Caliscelis and Asarcopus; Quayle 1938, O’Brien 1967) are found only in the west. Papagona, Fitchiella , and Bruchomorpha also occur in Mexico (Caldwell 1945, Bartlett et al. 2014), and a Bruchomorpha species (B. costaricensis Schmidt) is described from Costa Rica (Schmidt 1927). Emeljanov (1996c) divided Aphelonema into subgenera, with these subgenera subsequently recognized as full genera. The genera in the US are Aphelonema (4? species), Nenema (8 species), and Protrocha (12 species). For the time being, these three genera are treated on the same page.
de Freitas (2019) reviewed the neotropical caliscelid fauna. The work includes descriptions or redescriptions of many species and a key to Peltonotellini genera of the New World.
The fauna north of Mexico was revised by Doering (1939, 1941) (as part of Issidae).
Caliscelidae are largely grass feeders. The biology of Bruchomorpha oculata was described by Wilson and McPherson (1981). For this species, development from egg to adult averaged 68 days in a laboratory study, but the number of generations per year was unclear.
Contents
The classification of Caliscelidae north of Mexico
Family Caliscelidae Caliscelinae Amyot et Serville, 1843 Caliscelini Amyot et Serville, 1843
(= Peltonotidae Fieber, 1872 (unavailable) based on preoccupied name Peltonotus Mulsant & Rey, 1855)
Aphelonema Uhler, 1876 (Type species Aphelonema simplex Uhler, 1876)
= Peltonotus Mulsant & Rey 1855 (nec. Burmeister 1847) (Type species Peltonotus raniformis Mulsant & Rey 1855).
= Peltonotellus Puton 1886; replacement name for unavailable Peltonotus Mulsant & Rey 1855 (nec. Burmeister 1847); syn. by Melichar 1906: 36, 53; removed from syn. and redefined by Emeljanov 1996: 994 and Emeljanov 2008;
= Mushya Kato, 1933; syn. by Chan & Yang 1994: 8.
Asarcopus Horváth, 1921 (Type species Asarcopus palmarum Horváth, 1921).
Bruchomorpha Newman, 1838 (Type species Bruchomorpha oculata Newman, 1838).
Caliscelis de Laporte, 1833 (Type species Caliscelis heterodoxa de Laporte, 1833; jr. syn. of Fulgora bonellii Latreille, 1807).
Fitchiella Van Duzee, 1917b (Type species Naso robertsoni Fitch, 1856) (replacement name for unavailable Naso Fitch 1856)
Nenema Emeljanov 2008 Type species Peltonotellus bivittatus Ball, 1902) (species listed on Aphelonema page).
Papagona Ball, 1935a (Type species Papagona papoosa Ball, 1935a).
Protrocha Emeljanov, 1996 (Type species Aphelonema orbiculata Ball, 1935). (species listed on Aphelonema page)
Mesoamerican and Neotropical genera
Concepcionella Schmidt, 1927 (monotypic, Chile)
Itatiayana Metcalf, 1952 (=Itatiaya Schmidt, 1932 preoccupied) (monotypic, Brazil)
Ohausiella Schmidt, 1910 (Monotypic, Ecuador)
Paranaso Schmidt, 1932 (Monotypic, Brazil)
Plagiopsis Berg, 1883 (5 species, Argentina &?)
= Bergiella Melichar, 1906
= Bergiellula [sic] Metcalf, 1958
= Beriellula Strand, 1928
= Peripola Melichar, 1907
Plagiopsola Schmidt, 1927 (Monotypic, Costa Rica)
Semiperipola Schmidt, 1910 (Monotypic, Argentina)
Key to Genera of Caliscelidae north of Mexico
Note: This key includes Osbornia (Tropiduchidae: Elicini), since this genus can easily be mistaken for a caliscelid. The key still needs to be updated to account for Nenema and Protrocha, which will key to Aphelonema.
1. Front tibiae expanded, foliaceous …. 2
1’. Front tibiae not expanded or foliaceous …. 3
2. Front and middle tibiae expanded (slightly in F. robertsoni); head produced into weevil-like snout; frons, vertex, or pronotum pustulate; widespread (most common in southwest) ….Fitchiella Van Duzee
2’. Front tibiae and femora greatly expanded; head not produced into weevil-like snout; no pustules on head or thorax; sexually dimorphic, males black and yellow, females all tan; adventive in California …. Caliscelis de Laporte
3. Frons, vertex, or pronotum with distinct sensory pustules, usually in two or more rows; widespread distribution …. 4
3’. Pustules absent or indistinct; southwestern …. 6
4. Vertex longer than broad, southwestern …. Papagona Ball
4’ Vertex broader than long, widespread …. 5

Papagona succinea Ball, 1935 (ventral view of head, left); photo by Kimberley Shropshire (University of Delaware)
5. Head usually produced and snout-like; vertex crescent shaped, 5-6 times as broad as long …. Bruchomorpha Newman
5’. Head not produced snout-like; vertex usually less than 4 times as broad as long (A. decorata, A. simplex, and A. obscura excepted; these with median tablet of frons circular) ….Aphelonema Uhler

Aphelonema simplex Uhler, 1876 (left) and Bruchomorpha oculata Newman, 1838 (right); photos by Kimberley Shropshire.
6. Forewings touching medially, tightly fitted to body, venation not reticulate; frons slightly anteriorly projected; anterior margin of vertex rounded in dorsal view; usually orangish; adventive, on date palms in California … Asarcopus Horvath
6’. Forewings slightly separated medially, not touching body along all margin, venation often reticulate, especially distally; vertex varied, either lateral margins dorsally projected (O. cornuta) or medially angular in dorsal view (O. arborea); color dark with pale markings to mostly pale …. Osbornia Ball
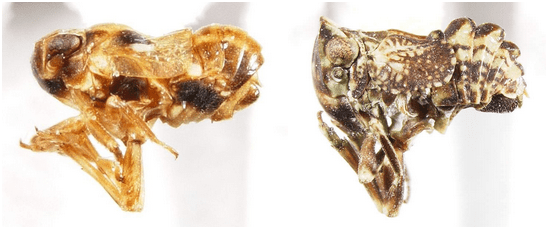
Asarcopus palmarum Horváth, 1921 (Caliscelidae) and Osbornia cornuta Ball, 1910 (Tropiduchidae Gaetuliini).
Higher classification
Caliscelidae Amyot & Audinet-Serville, 1843
Subfamily Caliscelinae Amyot & Audinet-Serville, 1843
Tribe Caliscelini Amyot & Audinet-Serville, 1843 (all Old World except adventive species in Asarcopus and Caliscelis)
Afronaso Jacobi, 1910
Ahomocnemiella Kusnezov, 1929
Annamatissus Gnezdilov & Bourgoin, 2014
Asarcopus Horváth, 1921
Bambusicaliscelis Chen & Zhang, 2011
Bolbonaso Emeljanov, 2007
Bruchoscelis Melichar, 1906
Calampocus Gnezdilov & Bourgoin, 2009
Caliscelis De Laporte, 1833, type genus
Chirodisca Emeljanov, 1996
Cylindratus Meng, Qin & Wang, 2020
Formibelle Sohail & Zhang, 2019
Formiscurra Gnezdilov & Viraktamath, 2011
Gelastissus Kirkaldy, 1906
Griphissus Fennah, 1967
Gwurra Linnavuori, 1973
Homocnemia Costa, 1857
Issopulex China & Fennah, 1960
Madaceratops Gnezdilov, 2011
Myrmissus Linnavuori, 1973
Nenasa Chan & Yang, 1994
Nubianus Gnezdilov & Bourgoin, 2009
Ordalonema Dlabola, 1980
Patamadaga Gnezdilov & Bourgoin, 2009
Populonia Jacobi, 1910
Reinhardema Gnezdilov, 2010
Rhinogaster Fennah, 1949
Rhinoploeus Gnezdilov & Bourgoin, 2009
Savanopulex Dlabola, 1987
Sphenax Gnezdilov & Bourgoin, 2009
Thaiscelis Gnezdilov, 2015
Ugandana Metcalf, 1952
Tribe Peltonotellini Emeljanov, 2008 (Old and New World)
Acromega Emeljanov, 1996 (monotypic, east Asia)
Aphelonema Uhler, 1876 (= Mushya Kato, 1933; 18 species, widespread nearly Holarctic)
Bergrothora Metcalf, 1952 (monotypic, Africa)
Bruchomorpha Newman, 1838 (= Embolonia Provancher, 1889; 25 species, Nearctic inc. Mesoamerica)
Ceragra Emeljanov, 1996 (3 species, middle Asia)
Concepcionella Schmidt, 1927 (monotypic, Chile)
Fitchiella Van Duzee, 1917 (= Naso Fitch, 1856; 8 species, Nearctic)
Homaloplasis Melichar, 1906 (monotypic, Mediterranean)
Itatiayana Metcalf, 1952 (Itatiaya Schmidt, 1932 preoccupied) (monotypic, Brazil)
Nenema Emeljanov, 1996 (8 species, Nearctic – widely distributed in the US, Canada and Mexico, except largely absent in the southeastern US)
Ohausiella Schmidt, 1910 (Monotypic, Ecuador)
Papagona Ball, 1935 (2 species SW USA)
Paranaso Schmidt, 1932 (Monotypic, Brazil)
Peltonotellus Puton, 1886 (type genus; 11 species, Palearctic)
Peripola Melichar, 1907 (Monotypic, Argentina)
Plagiopsis Berg, 1883 (4 species, Argentina &?)
Plagiopsola Schmidt, 1927 (Monotypic, Costa Rica)
Protrocha Emeljanov, 1996 (12 species, central US and Mexico)
Semiperipola Schmidt, 1910 (Monotypic, Argentina)
Subfamily Ommatidiotinae Fieber, 1875
Tribe Adenissini Dlabola, 1980 (southern Asia, Middle East, Northwest Africa)
subtribe Adenissina Dlabola, 1980
Adenissus Linnavuori, 1973, type genus
Perissana Metcalf, 1952
Raunolina Gnezdilov & Wilson, 2006
subtribe Bocrina Emeljanov, 1999
Bocra Emeljanov, 1999
subtribe Coinquendina Gnezdilov & Wilson, 2006
Coinquenda Distant, 1916
Delhina Distant, 1912
Lasonia Melichar, 1903
subtribe Pteriliina Gnezdilov & Wilson, 2006
Distantina Gnezdilov & Wilson, 2006
Phusta Gnezdilov, 2008
Pterilia Stål, 1859
Pterygoma Melichar, 1903
Tribe Augilini Baker, 1915 (tribe mostly tropical Asia and Indomalaya with few taxa in Afrotropics)
Anthracidium Emeljanov, 2013
Augila Stål, 1870
Augilina Melichar, 1914
Augilodes Fennah, 1963
Campures Gnezdilov, 2015
Cano Gnezdilov, 2011
Cicimora Emeljanov, 1998
Discote Emeljanov, 2013
Neosymplana Chen & Gong, 2020
Polychornum Gnezdilov, 2021
Pseudosymplanella Che, Zhang & Webb, 2009
Quizqueiplana† Bourgoin & Wang, 2015
Signoreta Gnezdilov & Bourgoin, 2009
Symplana Kirby, 1891
Symplanella Fennah, 1987
Symplanodes Fennah, 1987
Tubilustrium Distant, 1916
Youtuus Gong, Yang & Chen, 2018
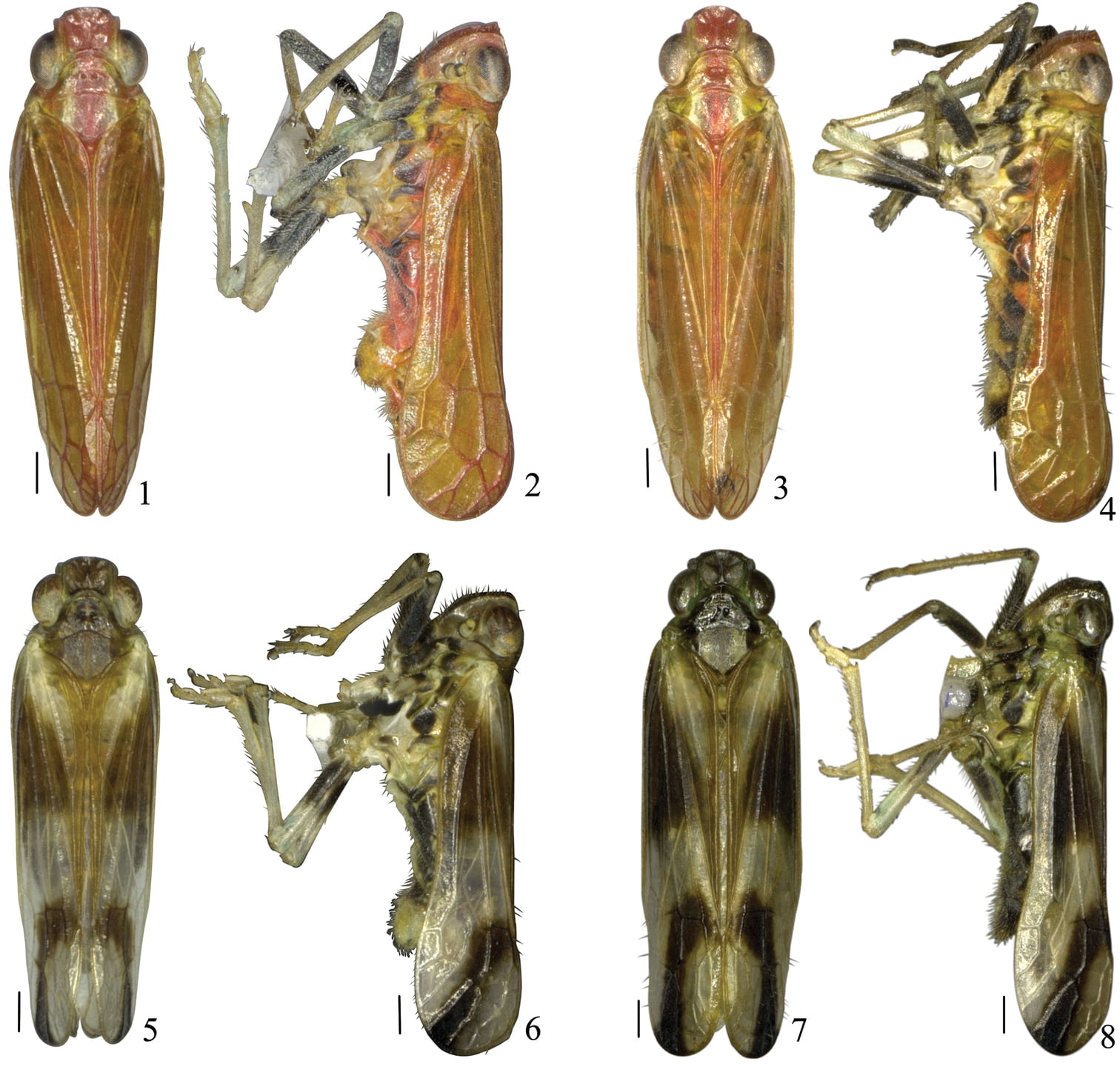
Youtuus erythrus Gong, Yang & Chen, 2018. 1 Male habitus, dorsal view 2 Male habitus, lateral view 3 Female habitus, dorsal view 4 Female habitus, lateral view; Youtuus strigatus Gong, Yang & Chen, 2918. 5 Male habitus, dorsal view 6 Male habitus, lateral view 7 Female habitus, dorsal view 8 Female habitus, lateral view. Scale bars: 0.5 mm.
Tribe Ommatidiotini Fieber, 1875 (widespread in Asia, including western Europe)
Ommatidiotus Spinola, 1839
References
Ball, E.D. 1935. The genus Bruchomorpha Newman (Homoptera-Fulgoridae). Bulletin of the Brooklyn Entomological Society 30: 197-203.
Bartlett, C.R., L.B. O’Brien & S.W. Wilson. 2014. A review of the planthoppers (Hemiptera: Fulgoroidea) of the United States. Memoirs of the American Entomological Society 50: 1-287.
Bourgoin, T. 2016. FLOW (Fulgoromorpha Lists on The Web): a world knowledge base dedicated to Fulgoromorpha. Version 8, updated 2016-09-15. http://flow.snv.jussieu.fr/ (accessed Sept. 15, 2016).
Caldwell, J.S. 1945. Notes on Issidae from Mexico (Homoptera: Fulgoroidea). Annals of the Entomological Society of America 38: 89-120.
de Freitas, A.S. 2019. Revisão taxonômica e filogenia dos Caliscelidae da região Neotropical (Insecta: Hemiptera). M.S. dissertação, programa de pós-graduação em biodiversidade e biologia evolutiva, universidade federal do rio de janeiro. Universidade Federal Do Rio de Janeiro Programa de Pós-Graduação em Biodiversidade E Biologia Evolutiva. Xvii+ 363 Pp.
de Freitas, A.S., C.H. Dietrich, & D. M. Takiya. 2020. Five new species of Caliscelidae (Insecta, Hemiptera) from Mexico and Panama, with additional redescriptions of little-known species. European Journal of Taxonomy 717: 27-69. https://doi.org/10.5852/ejt.2020.717.1097.
de Freitas, A.S., J.N. Zahniser, & D.M. Takiya. 2021. Review of the genus Papagona Ball, 1935 (Hemiptera: Caliscelidae) including a new Neotropical species. Zootaxa 5023(1): 107-120. https://doi.org/10.11646/zootaxa.5023.1.6.
de Laporte, F.L. 1833. Note sur un nouveau genre et un nouvel insecte homoptère (Caliscelis heterodoxa). Annales de la Société entomologique de France 2: 251-253.
Doering, K.C. 1939. A contribution to the taxonomy of the subfamily Issinae in America north of Mexico (Fulgoridae, Homoptera). Part III. University of Kansas Science Bulletin 26: 83-167.
Doering, K.C. 1941. A contribution to the taxonomy of the subfamily Issinae in America north of Mexico (Fulgoridae, Homoptera). Part IV. University of Kansas Science Bulletin 27: 185-233.
Emeljanov, A.F. 1996c. A new genus, Chirodisca gen. n., and new subgenera of the genus Aphelonema Uhl. (Homoptera, Fulgoroidea, Issidae). Entomologicheskoe Obozrenie 75(4): 834-835. [In Russian, English Translation 1996, 76: 993-994.]
Emeljanov, A.F. 1999. Notes on delimitation of families of the Issidae group with description of a new species of Caliscelidae belonging to a new genus and tribe (Homoptera, Fulgoroidea). Zoosystematica Rossica 8: 61-72.
Emeljanov, A.F. 2008. New species of the genus Peltonotellus Puton (Homoptera, Caliscelidae) from Kazakhstan, Middle and Central Asia. Tethys Entomological Research 16: 5-12.
Fennah, R.G. 1987. A recharacterisation of the Ommatidiotini (Hem. -Hom., Fulgoroidea, Issidae, Caliscelinae) with the description of two new genera. Entomologist’s Monthly Magazine 123: 243-247.
Gnezdilov, V.M. 2011. New and little known planthoppers of the subfamily Ommatidiotinae (Homoptera, Fulgoroidea, Caliscelidae) from Madagascar and South Asia. Entomological Review 91: 750-754.
Gnezdilov, V.M. 2014. A modern classification of the family Caliscelidae Amyot et Serville (Homoptera, Fulgoroidea). Entomological Review 94(2): 211–214.
Gnezdilov, V.M. & M.R. Wilson. 2006. Systematic notes on tribes in the family Caliscelidae (Hemiptera: Fulgoroidea) with description of new taxa from Palaearctic and Oriental regions. Zootaxa 1359: 1-30.
Hamilton, K.G.A. 2002. Homoptera (Insecta) in Pacific Northwest grasslands. Part 2 – Pleistocene refugia and postglacial dispersal of Cicadellidae, Delphacidae and Caliscelidae. Journal of the Entomological Society of British Columbia. 99: 33-80.
Hamilton, K.G.A. 2004. Leafhoppers and their relatives (Homoptera, Auchenorrhyncha) from the Canadian Great Plains. Arthropods of Canadian Grasslands. Biological Survey of Canada: Terrestrial Arthropods 101: 7-24.
Lawson, P.B. 1933. The genus Fitchiella (Homoptera, Fulgoridae). Bulletin of the Brooklyn Entomological Society. 28: 194-198.
Maw, H.E.L., R.G. Foottit & K.G.A. Hamilton. 2000. Checklist of the Hemiptera of Canada and Alaska, NRC Research Press, Ottawa, Canada.
Melichar, L. 1906. Monographie der Issiden. (Homoptera). Abhandlungen der K. K. Zoologisch-botanischen Gesellschaft in Wien 3: 1-327.
Metcalf, Z.P. 1958. General catalogue of the Homoptera. Fasc. IV. Fulgoroidea, Part 15. Issidae. North Carolina State University, Raleigh, NC. 561 pp.
O’Brien, L.B. 1967. Caliscelis bonellii (Latreille), a European genus of Issidae new to the United States (Homoptera: Fulgoroidea). Pan-Pacific Entomologist 43: 130 133.
Quayle, H.J. 1938. Insects of citrus and other subtropical fruits. ix + 583 pp.
Schmidt, E. 1927. Neue Zikaden-Gattungen und Arten. Archiv für Naturgeschichte (Berlin) 91: 147-160.
Urban, J.M. & J.R. Cryan. 2007. Evolution of the planthoppers (Insecta: Hemiptera: Fulgoroidea). Molecular Phylogenetics and Evolution 42: 556-772.
Wilson, S.W. & J.E. McPherson. 1981. Descriptions of the immature stages of Bruchomorpha oculata with notes on Laboratory rearing. Annals of the Entomological Society of America 74: 341-344.
Yang, C.T. & T.Y. Chang. 2000. The external male genitalia of Hemiptera (Homoptera – Heteroptera). Shih Way Publishing, Taichung.
Yeh, W.B., C.T. Yang, & C.F. Hui. 2005. A molecular phylogeny of planthoppers (Hemiptera: Fulgoroidea) inferred from mitochondrial 16S rDNA sequences. Zoological Studies 44: 519-535.