[back to Higher Classification of Delphacidae]
[back to A checklist of New World delphacid species]
Contents
Family Delphacidae Leach, 1815
Subfamily Delphacinae Leach, 1815
Tribe Delphacini Leach, 1815
Genus Megamelus Fieber, 1866: 519.
Type species (in original combination): Delphax notula Germar, 1830: 57.
Distribution
Widespread in Holarctic and Neotropical regions; most species in the Nearctic.
Recognized species
29 species are recognized in this genus as follows:
New World
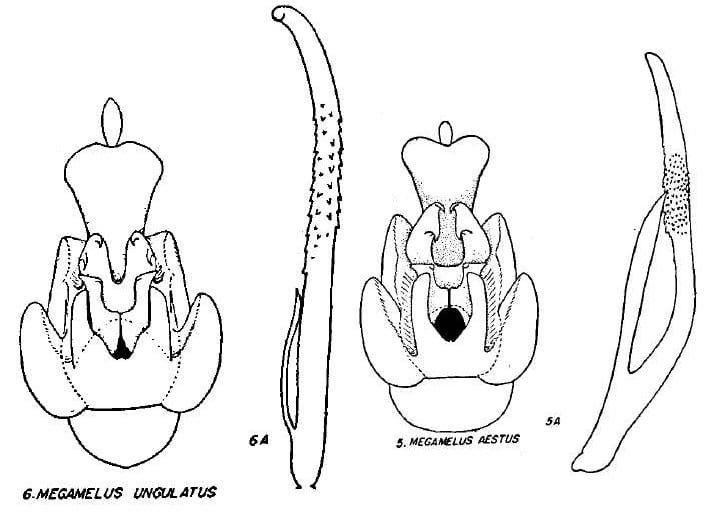
1 Megamelus aestus Metcalf, 1923: 202 – USA: Florida, Illinois, North Carolina, New Hampshire; Canada: Ontario, Quebec, Saskatchewan
2 Megamelus bellicus Remes Lenicov and Sosa, 2007: 801 – Argentina, Brazil, Peru
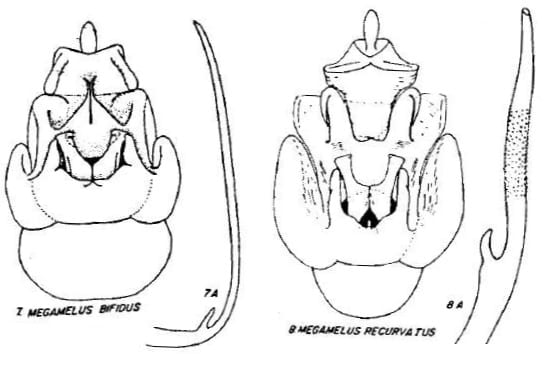
3 Megamelus bifidus Beamer, 1955: 34 – USA: Kansas, New York; Canada: Ontario
4 Megamelus bifurcatus Crawford, 1914: 612 – Brazil
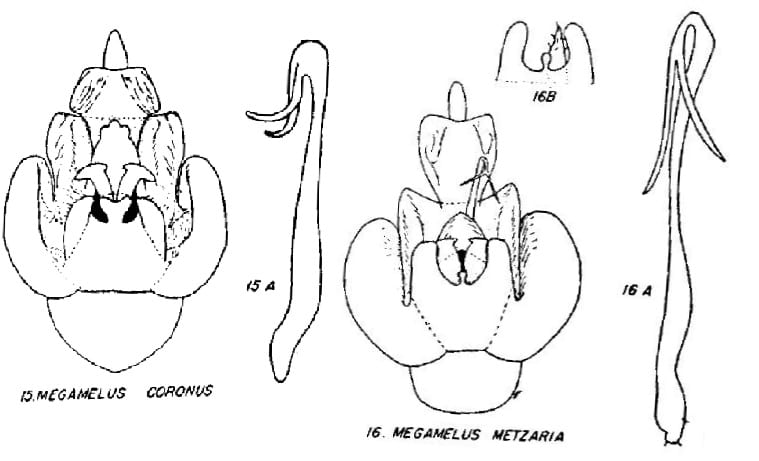
5 Megamelus coronus Beamer, 1955: 40 – USA: Texas
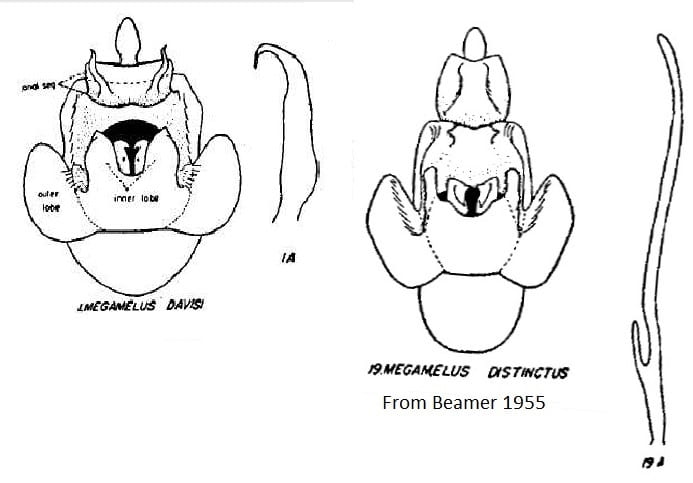
6 Megamelus davisi Van Duzee, 1897: 235 – USA: California, District of Columbia, Delaware, Florida, Georgia, Illinois, Kansas, Maryland, Michigan, Missouri, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Tennessee; also Hawaii, (adventive; Canada: Ontario
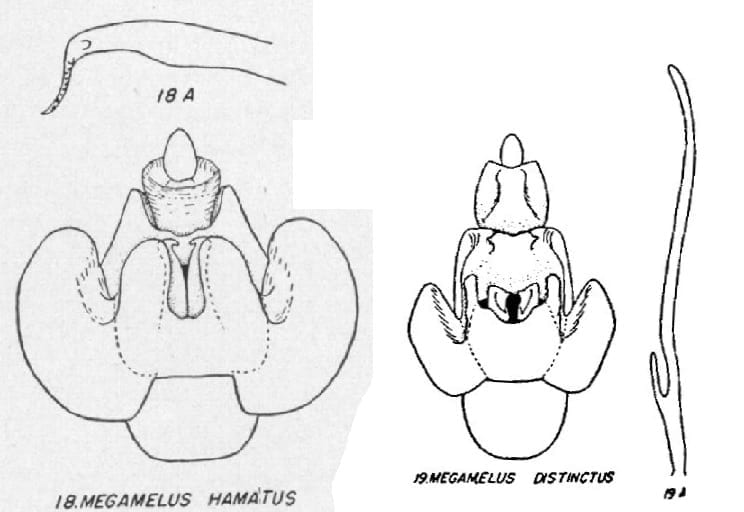
7 Megamelus distinctus Metcalf, 1923: 201 -USA: Connecticut, Illinois, Kansas, Michigan, Missouri, New York, North Carolina, Ohio; Canada: New Brunswick, Newfoundland, Nova Scotia, Ontario, Prince Edward Island, Quebec, Saskatchewan.
8 Megamelus electrae Muir, 1926: 28 – Argentina, Brazil, Dominican Republic, Puerto Rico, Trinidad and Tobago.
9 Megamelus falcatus Beamer, 1955: 39 – USA: Connecticut, New Hampshire, New York
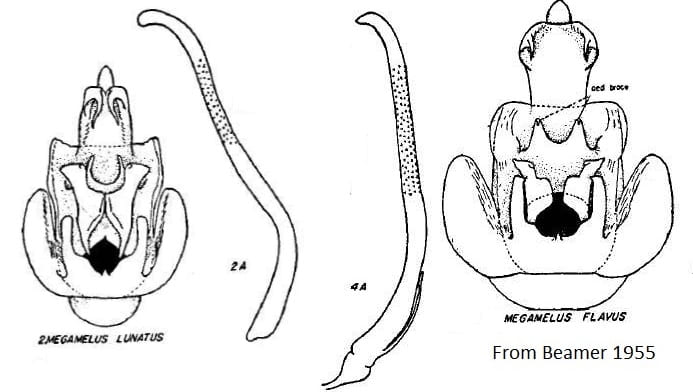
10 Megamelus flavus Crawford, 1914 – USA: Alaska, Colorado, , District of Columbia, Wyoming; Canada: Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland, Northwest Territories (Nunavut), Ontario, Quebec, Saskatchewan, Yukon; Mongolia, Russia (Kamchatka Region, Khabarovsk Territory, Sakhalin Region, Kurile Islands).
= Megamelus notulus flavus Crawford, 1914: 609.
= Megamelus flavus Crawford, 1914; status by Beamer 1955: 31.
= Megamelus anticostus Metcalf, 1923: 204; syn. by Beamer 1955: 31.
= Megamelus uncus Metcalf, 1923: 204; syn. by Beamer 1955: 31.
11 Megamelus gracilis Beamer, 1955: 35 – USA: Florida
12 Megamelus hamatus Beamer, 1955: 42 – USA: Delaware, Florida, Maryland, Virginia
13 Megamelus inflatus Metcalf, 1923: 203 – USA: New York; CAN: Nova Scotia, Quebec
14 Megamelus iphigeniae Muir, 1926: 28 – Argentina, Brazil, Panama (Canal Zone)
15 Megamelus lobatus Beamer, 1955: 38 – USA: Alabama, Connecticut, Delaware, Florida, Georgia, Louisiana, Maryland, Mississippi, New Hampshire, New Jersey, North Carolona, Texas
16 Megamelus longicornis (Dozier, 1922: 76) – USA: Louisiana, Mississippi, Texas
17 Megamelus lunatus Beamer, 1955: 30 – USA: Delaware, Illinois, Kansas, New York; Canada: British Columbia, Ontario, Quebec
18 Megamelus metzaria Crawford, 1914: 611 – USA: Illinois, Kansas, Michigan, Missouri, New Hampshire, New York, North Carolina, Wisconson, Wyoming; Canada: British Columbia(?), Manitoba, New Brunswick, Nova Scotia, Ontario, Prince Edward Island, Quebec, Saskatchewan
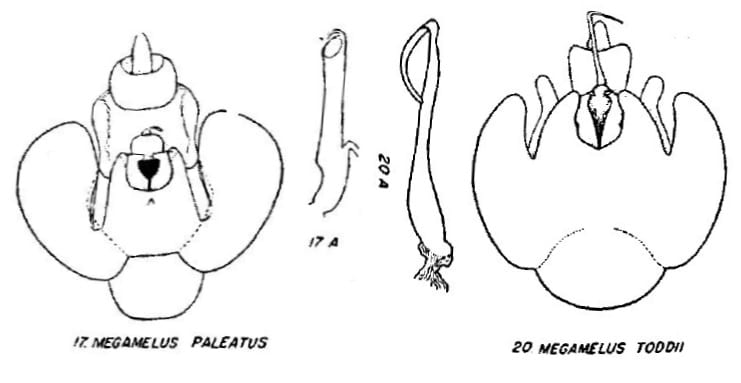
19 Megamelus palaetus (Van Duzee, 1897: 232) – USA: District of Columbia, Delaware, Florida, Georgia, Illinois, Louisiana, Mississippi, New York, North Carolina, South Carolina, Texas
20 Megamelus recurvatus Beamer, 1955: 34 – USA: Maine; Canada: Manitoba, Ontario
21 Megamelus scutellaris Berg, 1883: 235 (“waterhyacinth planthopper“)- Argentina, Brazil, Peru, Uruguay (candidate for introduction into Florida for biocontrol of water hyacinth; recently introduced into Louisiana, Florida and California)
22 Megamelus timehri Muir, 1919: 36 – Argentina, Guyana
23 Megamelus toddi Beamer, 1955: 43 – USA: Florida, Louisiana
24 Megamelus trifidus Beamer, 1955: 35 – USA: Alabama, Florida
25 Megamelus ungulatus Beamer, 1955: 33 – USA: Connecticut, Delaware, Florida, GeorgiaNorth Carolina, New Jersey, New York
Old World (see also Megamelus flavus above)
Palearctic
1Megamelus discrepans Haupt, 1930: 155 – Italy
2 Megamelus leptus Fieber, 1878: 279 – Austria, Czech Republic, Germany, Italy
3 Megamelus notula (Germar, 1830: 57) – Widespread: Europe, Russia, Japan (Honshu), Mongolia, Azerbaijan, Estonia, Kazakhstan, Latvia, Ukraine
Australia
1 Megamelus leimonias (Kirkaldy, 1907: 159) – Australia: Queensland (see Insects of Australia)
Plant associations
Species of this genus are associated with aquatic and semiaquatic plants in various families.
Megamelus bellicus – Pontederia cordata, P. rotundifolia, Eichhornia crassipes, E. azurea (Pontederiaceae); Echinodorus grandiflorum (Alismathaceae) and L. laevigatum (Hydrocharitaceae).
Megamelus capnodes – Carex riparia (Cyperaceae)
Megamelus davisi – water lily (Nymphaea spp.), American white water lily (Nymphaea odorata Aiton as Castalia odorata); pond lily (Nuphar advena; Nymphaeaceae); Pontederia cordata (Pontederiaceae); alligatorweed (Alternanthera philoxeroides: Amaranthaceae)
Megamelus electrae – Eichhornia azurea
Megamelus iphigeniae – Eichhornia azurea, Eichhornia crassipes, Pontederia parviflora
Megamelus lobatus – Spartina patens (Poaceae Eragrostideae Chloridoideae)
Megamelus metzaria – Spartina pectinata (Poaceae Eragrostideae Chloridoideae)
Megamelus notula – Carex spp. (C. acutiformis, C. acuta, C. rostrata, C. nigra, C. disticha, etc.), Juncus spp., Carex lasiocarpa Ehrh. (woollyfruit sedge), Carex riparia Curtis.
Megamelus paleatus – Eichornia crassipes; Pontederia cordata L (Pontederiaceae); broadleaf arrowhead – Sagittaria latifolia (Alismataceae), Paspalum sp. (Poaceae); Jatropha integerrima; Euphorbiaceae; Laurel;
Megamelus scutellaris, M. electrae – water hyacinth, Eichhornia crassipes (Pontederiaceae)
Megamelus timehri – Limnobium laevigatum (Hydrocharitaceae)
Host information compiled from Wilson et al. 1994, Holzinger et al. 2003, Sosa et al. 2007; See Also host associations reported on FLOW.
Economic Importance
Megamelus scutellaris is being used as a biocontrol agent of water hyacinth, Eichhornia crassipes Mart. (Solms) (Pontederiales: Pontederiaceae) (USEPA 2010).
Megamelus davisi has been inadvertently introduced into Hawaii.
Recognition
See Beamer (1955) for identification of North American species; Sosa et al. (2007) for Neotropical species. I am aware of at least 1 undescribed
Central American species.
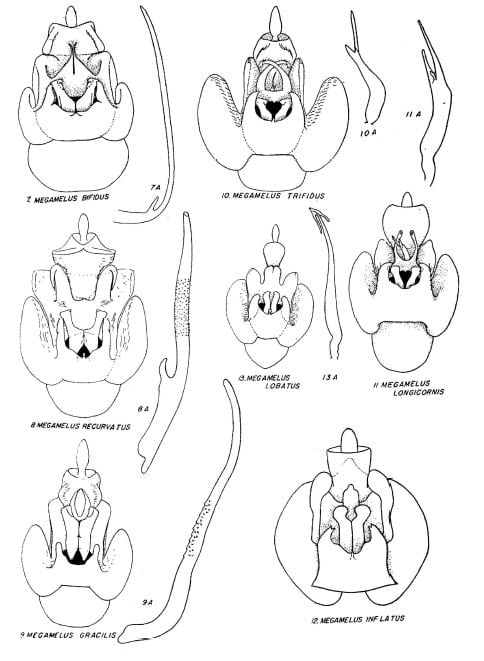
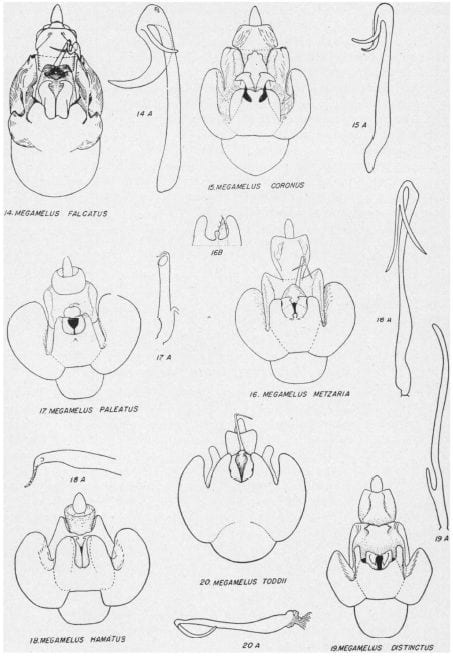
Key to the species of Megamelus, modified slightly from from Beamer (1955)
1. Male anal segment with processes … 2
1’. Male anal segment without processes … 5
2. Anal processes projecting anteriorly … 3
2’. Anal processes projecting posteriorly … Megamelus davisi
3. Apices of aedeagal brace turned ventrad …4
3’. Apices of aedeagal brace not turned ventrad … Megamelus lunatus
4. Aedeagus with a large sword-like process near base … Megamelus notulus (not a North America species)
4’. Aedeagus without such a process near base … Megamelus flavus
5. Aedeagel brace deeply split or excavated at apex …. 6
5’. Aedeagal brace not deeply split or excavated at apex …12
6. Sides of excavation with processes … 7
6’. Sides of excavation without processes … 8
7. With processes on inside of arms of excavation …Megamelus aestus
7’. With processes on outside of arms of excavation … Megamelus ungulatus
8. Sides of excavation almost touching … Megamelus bifidus
8’. Sides of excavation not almost touching … 9
9. Sides of aedeagel brace sharply recurved on outer third, apex sharp …Megamelus recurvatus
9’. Sides of aedeagal brace not sharply recurved, apices not sharp …10
10. Aedeagus trifid at apex …11
10’. Aedeagus not trifid at apex … Megamelus gracilis
11. Sides of aedeagal brace flat, bifid at apex … Megamelus trifidus
11’. Sides of aedeagal brace curved dorsally at tip, not bifid … Megamelus longicornis
12. Lobes at sides of styles bifid … Megamelus inflatus
12’. Lobes at sides of styles not bifid … 13
13. Apex of aedeagus bifid … 14
13’. Apex of aedeagus not bifid … 17
14. Bifid apex of shaft recurved …. 15
14’. Bifid apex of shaft not recurved …. Megamelus lobatus
15. Processes at apex of aedeagus about same width …. 16
15’. One process at apex of shaft much longer than other …. Megamelus falcatus
16. Apex of aedeagal brace trilobed …. Megamelus coronus
16’. Apex of aedeagal brace more or less pointed … Megamelus metzaria
17. Aedeagus long, cylindrical or flat … 18
17’. Aedeagus short, tubular, with sinuate process at apex … 19
18. Aedeagus flat, apical third sharply narrowed, bent at almost right angles to shaft … Megamelus hamatus
18’. Aedeagus long and narrow with a heavy short process near base … Megamelus distinctus
19. Aedeagus tapering from base to tip … Megamelus toddi
19’. Aedeagus not tapering from base to tip … Megamelus paleatus
Key to Megamelus species of South America (from Sosa et al. 2007, figure references to that paper) (note: there is one fairly common undescribed species from Mesoamerica)
1. Male anal segment with pair of asymmetrical processes arising near or just below posterior angle (Figs. 10 and 25). Aedeagus with two apical processes curved on each side (Figs. 11 and 26). Ovipositor long (Figs. 15 and 30) … 2
1’. Male anal segment without processes (Fig. 39), or with two symmetrical processes arising on base. Aedeagus with one process, or if two, not spiniform. Ovipositor short (Fig. 44) … 3
2. Processes of male anal segment long, slender, curved upward and projecting inwards just below posterior angle. Aedeagus tubular, processes projected toward base (Fig. 11). Pygofer with sclerotized area bearing a pair of small, sharp-pointed processes between inner lobes (Fig. 8). Female: valvifer VIII with inner margin produced and truncate at base (Fig. 14). Frons with one narrow light stripe on the apex, macropterous tegmina hyaline with only one fuscous mark on apex of clavus (Fig. 4) … M. bellicus Remes Lenicov & Sosa 2007.
2’. Processes of male anal segment stronger, curved in opposing directions (left dorsad, right ventrad) (Fig. 25). Aedeagus asymmetric with paired apical processes, one directed cephalad, other caudad; plus small dorsal furcate projection near middle (Fig. 26). Pygofer with lobe-like process between inner lobes (Fig. 23). Female: valvifer VIII with inner margin rounded at base (Fig. 29). Frons uniformly colored. Macropterous tegmina heavily infuscated on clavus and apical area (Fig. 19) … M. electrae Muir
3. Male anal segment with two small symmetrical processes (Fig. 53). Aedeagus with one apical closely curved process dorsobasally directed (Fig. 54). Pygofer with a small peg-like projection on ventral surface between inner lobes (Fig. 51). Small species: 3.4 mm. Vertex short, slightly longer than wide. Frons short, with median carina forked below anterior margin of eye. Tegmina hyaline (Fig. 47) … M. timehri Muir
3’. Anal segment without processes. Aedeagus with one or two lateral apical processes. Pygofer without processes between inner lobes. Median or large species: 3.8-4.2 mm. Vertex and frons longer, median carina of frons forked above anterior margin of eye. Tegmina infuscated (Fig. 33) … 4
4. Male anal segment collar like, not pointed. Aedeagus with two apical processes. Pygofer inner lobes rectangular in outline. Dorsal margin of diaphragm medially rectangularly produced. Female: sternite VII finely denticulated medially. Second valvula with conspicuous, truncated, and dorsally denticulated teeth on apical half. Frons with two transverse light stripes … M. scutellaris Berg
4’. Male anal segment slightly pointed on medioventral margin of apex (Fig. 35). Aedeagus with one apical process (Fig. 40). Pygofer inner lobes sinuous in outline (Fig. 37). Dorsal margin of diaphragm medially widely rounded produced (Fig. 36). Female: sternite VII with a medial tongue-like projection directed caudally (Fig. 42). Second valvula with small pointed teeth (Fig. 44). Frons brown and uniformly colored … M. iphigeniae Muir
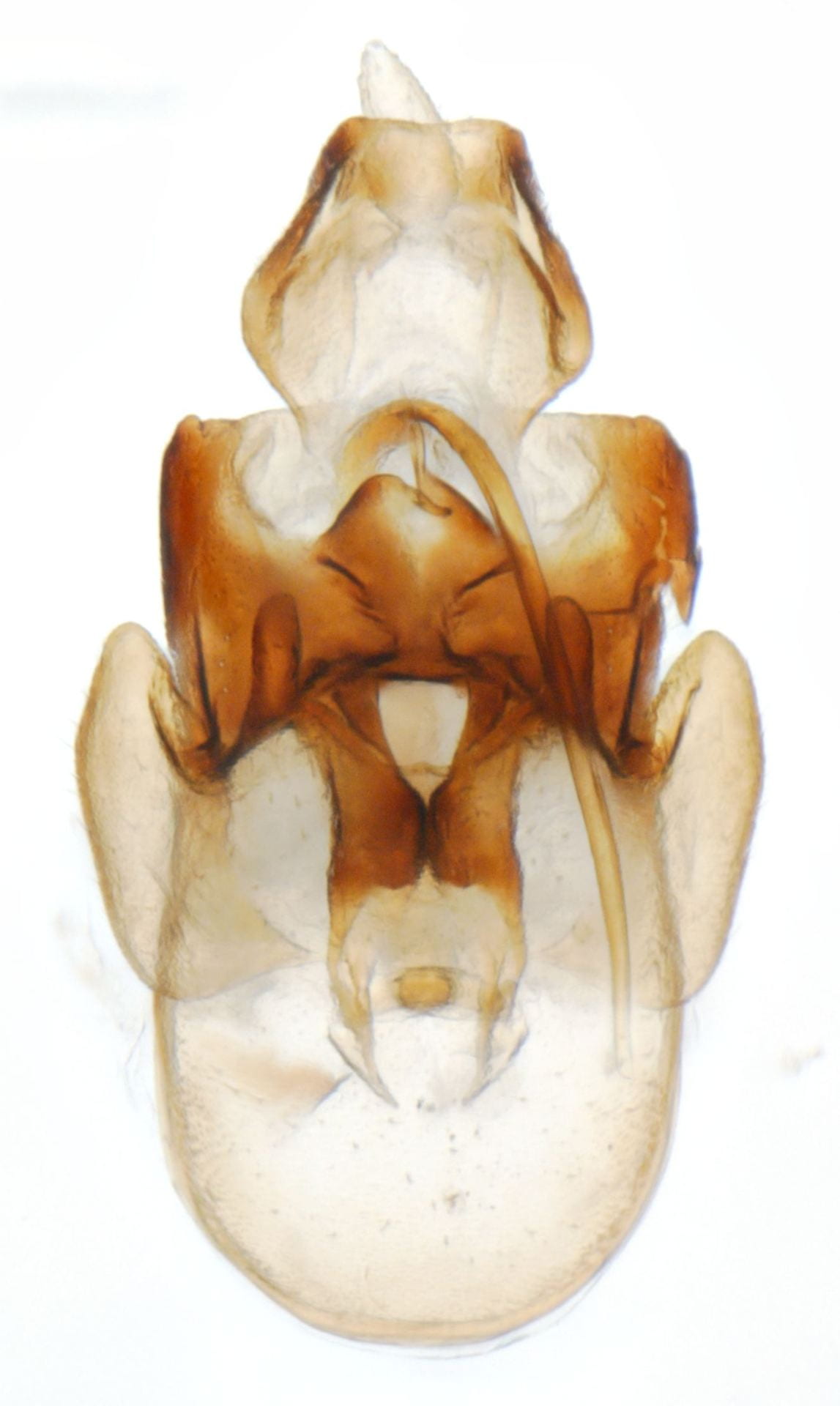
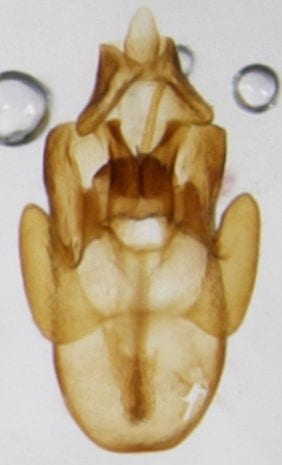
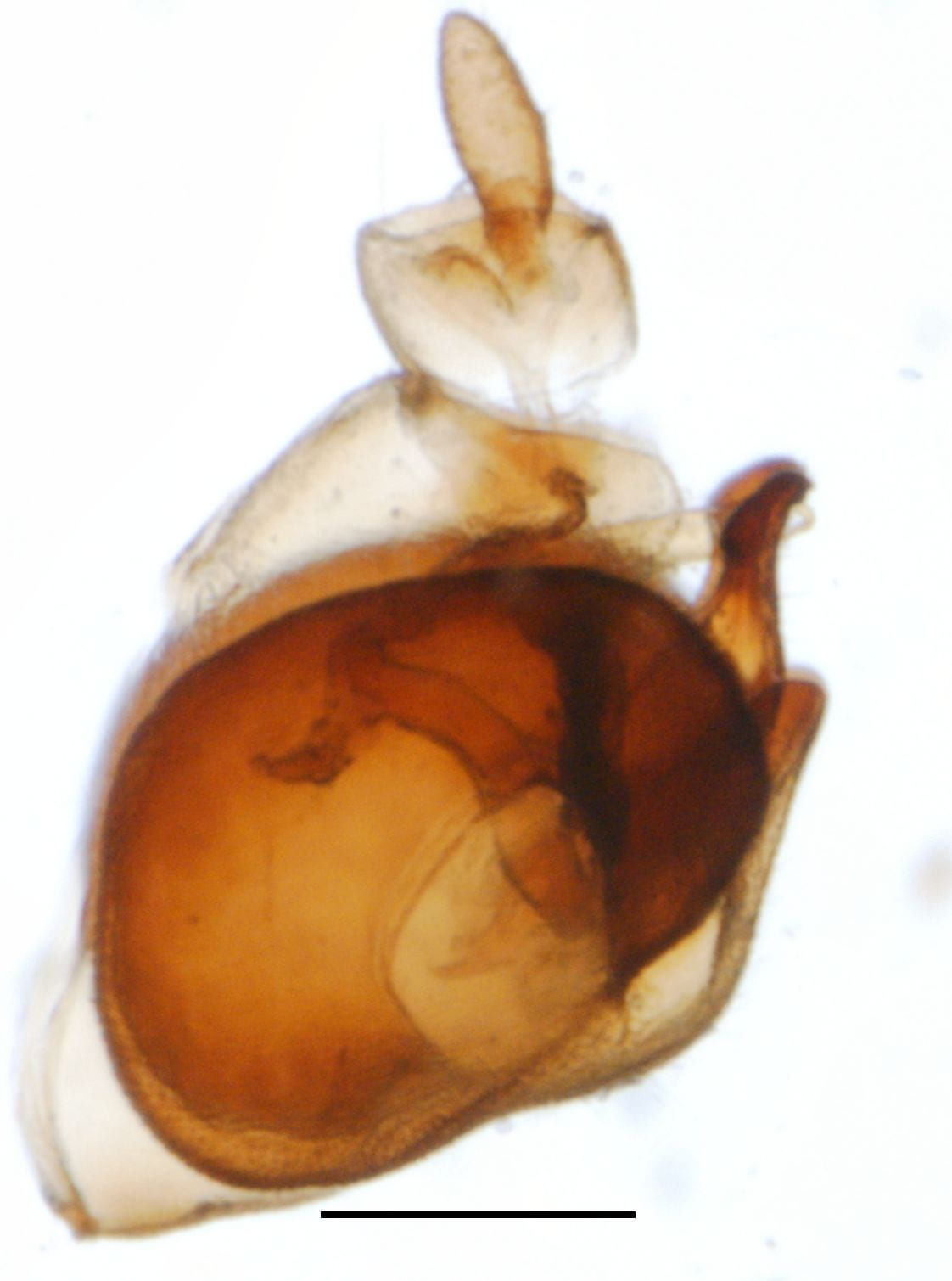
Megamelus notula
Megamelus davisi – male (dark form; there are also much lighter specimens)

Megamelus davisi
Megamelus distinctus – male
Megamelus distinctus – Female
Megamelus flavus
Megamelus lobatus – male
Megamelus metzaria – male
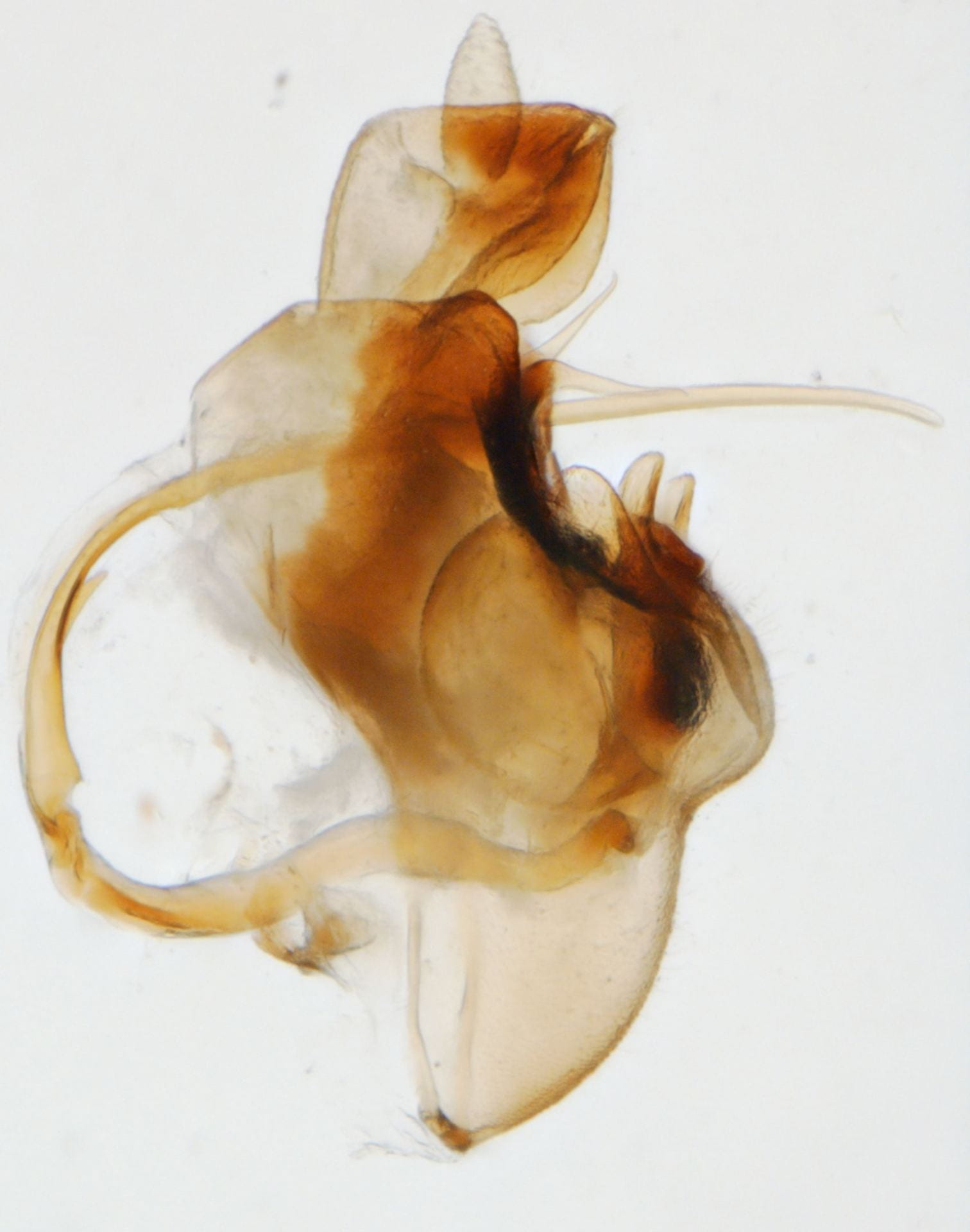
 Megamelus paleatus (A common species at lights in the SE USA).
Megamelus paleatus (A common species at lights in the SE USA).
Plates from Beamer (1955)
Online resources
American Insects
EOL
FLOW
Discover Life
Bugguide
GBIF
3I Interactive Keys and Taxonomic Databases (Dmitry Dmitriev)
Insects of Australia (Megamelus leimonias)
Gernot Kunz (Megamelus notula)
British Bugs (Megamelus notula)
BOLD.
Web Images
Flickr (search=Megamelus)
Molecular resources
At this time, Genbank provides some sequence data for 4 Megamelus species. BOLD now has data for several species (10 species, 4 with public data) and a lot of specimens (updated Oct. 2018, checked 31 Oct 2020).
References
(more to add, along with links to BHL)
Asche, M. 1997. A review of the systematics of Hawaiian planthoppers (Hemiptera: Fulgoroidea). Pacific Science 51 (4): 366-376.
Au, S. H. 1941. Megamelus davisi infesting water lily in Hawaii. Journal of Economic Entomology 34: 415.
Barringer, L. E. and C. R. Bartlett. 2018. Pennsylvania planthoppers (Hemiptera: Auchenorrhyncha: Fulgoroidea): relative abundance and incidental catch using novel trapping methods. Insecta Mundi 0661: 1–31.
Bartlett, C. R., L. B. O’Brien and S. W. Wilson. 2014. A review of the planthoppers (Hemiptera: Fulgoroidea) of the United States. Memoirs of the American Entomological Society 50: 1-287.
Beamer, R. H. 1955. A revision of the genus Megamelus in America north of Mexico (Homoptera, Fulgoridae, Delphacinae). Journal of the Kansas Entomological Society 28(1): 29-46. (this link is to part 2 in JSTOR; part 1 is here)
Berg, C. 1883b. Addenda et emendanda ad Hemiptera Argentina (Continuatio). Anales de la Sociedad Científica Argentina 16: 231-241.
Crawford, D. L. 1914. A contribution toward a monograph of the homopterous insects of the family Delphacidae of North and South America. Proceedings of the United States National Museum 46: 557-640, plus 6 plates.
Denno, R. F., G. K. Roderick and K. L. Olmstead. 1991. Density-related migration in planthoppers (Homoptera: Delphacidae): the role of habitat persistence. American Naturalist 138(6): 1513-1541
Dozier, H. L. 1922. A synopsis of the genus Stenocranus, and a new species of Mysidia (Homoptera). Ohio Journal of Science 22: 69-82.
Fieber, F. X. 1866b. Grundzüge zur generischen Theilung der Delphacini. Verhandlungen der Kaiserlich-Königlichen Zoologish-botanischen Gesellschaft in Wien 16: 517-534.
Fieber, F. X. 1878. Les Cicadines d’Europe d’après les originaux et les publications les plus récentes. Troisième partie: Descriptions des espèces. Revue et Magasin de Zoologie pure et appliquée (Ser. 3) 6: 270-308.
Germar, E. F. 1830. Species Cicadarium enumeratae et sub genera distributae. Thon’s Entomologisches Archiv 2(2): 1-57.
Goode, A.B.C., C. R. Minteer, P. W. Tipping, B. K. Knowles, R. J. Valmonte, J. R. Foley and L. A. Gettys. 2019. Small-scale dispersal of a biological control agent – Implications for more effective releases. Biological Control 132: 89–94. https://doi.org/10.1016/j.biocontrol.2019.01.016
Haupt, H. 1930. Drei neue Homoptera-Cicadina aus Ligurien (Italien). Mitteilungen der Deutschen Entomologischen Gesellschaft 1: 153-159.
Holzinger, W. E., I. Kammerlander and H. Nickel. 2003. Fulgoromorpha, Cicadomorpha excluding Cicadellidae. Volume 1. The Auchenorrhyncha of Central Europe. Brill Academic Publishing, Leiden, Netherlands. [Host information for M. notula]
Hopper, J. V., P. D. Pratt, K. F. McCue, M. J. Pitcairn, P. J. Moran and J. D. Madsen. 2017. Spatial and temporal variation of biological control agents associated with Eichhornia crassipes in the Sacramento-San Joaquin River Delta, California. Biological Control 111: 13–22. doi: 10.1016/j.biocontrol.2017.1005.1005. [includes Megamelus scutellaris (Delphacidae)]
Kirkaldy, G. W. 1907d. Leafhoppers supplement. (Hemiptera). Bulletin. Hawaiian Sugar Planters’ Association Experiment Station. Division of Entomology 3: 1-186.
Kontkanen, P. 1958. Notes on some Fulgorids collected in Canada by Professor Håkon Lindberg during the summer of 1956 (Homoptera: Fulgoroidea). Annales Entomologici Fennici 24: 141-145.
Leach, W. E. 1815. Entomology. The Edinburgh Encyclopedia 9: 57-172. [Delphacidae on p. 125].
Mariani, R., A. Sosa and A. M. M. de Remes Lenicov. 2007. Megamelus bellicus (Hemiptera: Delphacidae): immature stages and biology. Revista de la Sociedad Entomologica Argentina 66 (3-4): 189-196.
Maw, H.E.L., R. G. Foottit, K.G.A. Hamilton and G.G.E. Scudder. 2000. Checklist of the Hemiptera of Canada and Alaska. NRC Research Press, Ottawa.
Metcalf, Z. P. 1923a. A key to the Fulgoridae of Eastern North America with descriptions of new species. Journal of the Elisha Mitchell Scientific Society 38(3): 139-230, plus 32 plates.
Metcalf, Z. P. 1943. General Catalogue of the Hemiptera. Fascicle IV, Fulgoroidea, Part 3, Araeopidae (Delphacidae). Smith College, Northhampton, Massachusetts.
Metcalf, Z. P. 1949. The redescription of twenty-one species of Areopidae described in 1923. Journal of the Elisha Mitchell Scientific Society 65(1): 48-60 plus, 4 plates.
Moore, G. A. 1950a. Catalogus des hémiptères de la province de Québec. Le Naturaliste Canadien 77: 233-271.
Moore, G. A. 1950b. Check-list of Hemiptera of the province of Quebec. Contributions de l’Institut de Biologie de l’Université de Montréal. 26: 1-49.
Moran, P. J., M. J. Pitcairn and B. Villegas. 2016. First establishment of the planthopper, Megamelus scutellaris Berg, 1883 (Hemiptera: Delphacidae), released for biological control of water hyacinth in California. Pan-Pacific Entomologist 92(1): 32-43.
Muir, F.A.G. 1919b. Some New American Delphacidae. Canadian Entomologist 51(2): 35-39.
Muir, F.A.G. 1926b. Contributions to our knowledge of South American Fulgoroidea (Homoptera). Part I. The Family Delphacidae. Experiment Station of the Hawaiian Sugar Planters’ Association, Entomological Series, Bulletin 18:1-51, plates 1-5.
Oman, P. W. 1947. The types of Auchenorrhynchous Homoptera in the Iowa State College Collection. Iowa State College Journal of Science 21: 161-228.
Paiero, S. M., S. A. Marshall and K.G.A. Hamilton. 2003. New records of Hemiptera from Canada and Ontario. Journal of the Entomological Society of Ontario 134: 115-129
Scudder, G.G.E. 1964. Studies on the Canadian and Alaskan Fulgoromorpha (Hemiptera). II. The genus Megamelus Fieber (Delphacidae). Canadian Entomologist 96(6):813-820.
Sosa, A. J. , A. M. M. de Remes Lenicov, R. Mariani and H. A. Cordo. 2005. Life history of Megamelus scutellaris with description of immature stages (Hemiptera: Delphacidae). Annals of the Entomological Society of America 98(1): 66-72.
Sosa, A. J., H. A. Cordo and J. Sacco. 2007. Preliminary evaluation of Megamelus scutellaris Berg (Hemiptera: Delphacidae), a candidate for biological control of waterhyacinth. Biological Control 42: 129–138.
Sosa, A. J., A.M.M. de Remes Lenicov and R. Mariani. 2007. Species of Megamelus (Hemiptera: Delphacidae) Associated with Pontederiaceae in South America. Annals of the Entomological Society of America 100(6): 798-809. https://academic.oup.com/aesa/article/100/6/798/8371
Sosa, A. J., A.M.M. de Remes Lenicov, R. Mariani and H. A. Cordo 2004. Redescription of Megamelus scutellaris Berg (Hemiptera: Delphacidae), a candidate for biological control of water hyacinth. Annals of the Entomological Society of America 97(2): 271-275.
Tipping, P. W., T. D. Center, A. J. Sosa and F. A. Dray. 2010. Host specificity assessment and potential impact of Megamelus scutellaris (Hemiptera: Delphacidae) on waterhyacinth Eichhornia crassipes (Pontederiales: Pontederiaceae). Biocontrol Science and Technology 21(1): 75-87.
Tipping, P. W., T. D. Center, A. J. Sosa, and F. A. Dray. 2011. Host specificity assessment and potential impact of Megamelus scutellaris (Hemiptera: Delphacidae) on waterhyacinth Eichhornia crassipes (Pontederiales: Pontederiaceae). Biocontrol Science and Technology 21(1): 75-87.
Tipping, P. W., A. Sosa, E. N. Pokorny, J. Foley, D. C. Schmitz, J. S. Lane, L. Rodgers, L. Mccloud, P. Livingston-Way, M. S. Cole and G. Nichols. 2014. Release and establishment of Megamelus scutellaris (Hemiptera: Delphacidae) on Waterhyacinth in Florida. Florida Entomologist 97(2): 804-806.
Tsai, J. H. and F. W. Mead, 1982. Rotary net survey of homopterans in palm plantings in south Florida. Journal of Economic Entomology 75(5): 809-812.
USDA. 2010. Field Release of Megamelus scutellaris, Berg (Hemiptera: Delphacidae), for Biological Control of Water Hyacinth Eichhornia crassipes Mart. (Solms) (Pontederiales: Pontederiaceae) in the Continental United States. Environmental Assessment, July 2009. Riverdale, MD. 23 pp. + indices.
Van Duzee, E. P. 1897a. A Preliminary Review of the North American Delphacidae. Bulletin of the Buffalo Society of Natural Sciences 5(5): 225-261.
Wheeler, A. G. and E. R. Hoebeke. 2008. Conomelus anceps (Germar) (Hemiptera: Fulgoromorpha: Delphacidae) new to North America, with records of four other delphacid planthoppers new to Newfoundland. Proceedings of the Entomological Society of Washington 110(2): 265-283.
Wilson, S. W. 1988. Delphacidae of Alaska (Homoptera: Fulgoroidea). Great Basin Naturalist Memoirs 12: 335-343. pdf
Wilson, S. W. 1992. The Delphacidae of Yukon Territory, Canada (Homoptera: Fulgoroidea). Insecta Mundi 6(2): 79-100.
Wilson, S. W. 1997. Delphacid planthoppers (Homoptera: Fulgoroidea: Delphacidae) of the Yukon. Pp. 377-385. In: H. V. Danks and J. A. Downes (eds.). Biological Survey of Canada (Terrestrial Arthropods), Ottawa, Canada.
Wilson, S. W. and J. E. McPherson. 1979. The first record of Megamelus palaetus in Illinois (Homoptera: Fulgoroidea: Delphacidae). Great Lakes Entomologist 12(4): 227.
Wilson, S. W. and J. E. McPherson. 1980. Keys to the planthoppers, or Fulgoroidea of Illinois (Homoptera). Transactions of the Illinois State Academy of Science 73(2): 1-61.
Wilson, S. W. and J. E. McPherson. 1980. A list of the Fulgoroidea (Homoptera) of southern Illinois. Great Lakes Entomologist 13(1): 25-30.
Wilson, S. W. and J. E. McPherson. 1980. The distribution of the Fulgoroidea of the Eastern United States (Homoptera). Transactions of the Illinois State Academy of Science 73(4): 7-20.
Wilson, S. W. and J. E. McPherson. 1981. Life history of Megamelus davisi with descriptions of immature stages. Annals of the Entomological Society of America 74(4): 345-350.
Wilson, S. W. and J. E. McPherson. 1981. Ontogeny of the tibial spur in Megamelus davisi (Homoptera: Delphacidae) and its bearing on delphacid classification. Great Lakes Entomologist 14(1): 49-50.
Wilson, S.W., C. Mitter, R.F. Denno and M.R. Wilson. 1994. Evolutionary patterns of host plant use by delphacid planthoppers and their relatives. In: R.F. Denno and T.J. Perfect, (eds.). Planthoppers: Their Ecology and Management. Chapman and Hall, New York. Pp. 7-45 & Appendix [host references in appendix].