2009-present a) Solid-state NMR studies on structure and dynamics of HIV-1 capsid proteins; b) Solid-state NMR methodology development in biological solids; c) Solid-state NMR studies on vanadium (V) containing proteins and bioinorganic solids.
2007-2009 a) Solid state NMR studies on macromolecular and supra-molecular systems; b) Studies of structure and dynamics of ion-conducting solid polymer electrolytes
2003-2007 Heteronuclear & Homonuclear recoupling techniques and their applications in solid state NMR
2001-2003 Experimental Measurement and Theoretical Computation of Chemical Shift Tensor in solid state NMR.
Featured Researches
A. Proton CSA determination in protonated protein assemblies

J. Am. Chem. Soc., 2013, 135 (4), pp 1358–1368 DOI: 10.1021/ja3084972
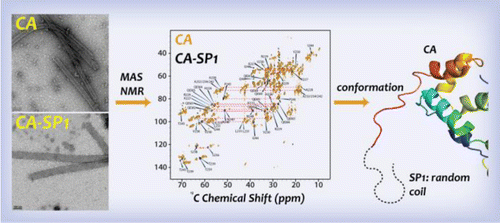
B.Structural plasticity, dynamics and SP1 conformation of HIV-1 CA assemblies
J. Am. Chem. Soc., 2013, 135, pp 17793-17803 DOI: 10.1021/ja406907h
A key stage in HIV-1 maturation toward an infectious virion requires sequential proteolytic cleavage of the Gag polyprotein leading to the formation of a conical capsid core that encloses the viral RNA genome and a small complement of proteins. The final step of this process involves severing the SP1 peptide from the CA-SP1 maturation intermediate, which triggers the condensation of the CA protein into the capsid shell. The details of the overall mechanism, including the conformation of the SP1 peptide in CA-SP1, are still under intense debate. In this report, we examine tubular assemblies of CA and the CA-SP1 maturation intermediate using magic angle spinning (MAS) NMR spectroscopy. At magnetic fields of 19.9 T and above, outstanding quality 2D and 3D MAS NMR spectra were obtained for tubular CA and CA-SP1 assemblies, permitting resonance assignments for subsequent detailed structural characterization. Dipolar- and scalar-based correlation experiments unequivocally indicate that SP1 peptide is in a random coil conformation and mobile in the assembled CA-SP1. Analysis of two CA protein sequence variants reveals that, unexpectedly, the conformations of the SP1 tail, the functionally important CypA loop, and the loop preceding helix 8 are modulated by residue variations at distal sites. These findings provide support for the role of SP1 as a trigger of the disassembly of the immature CA capsid for its subsequent de novo reassembly into mature cores and establish the importance of sequence-dependent conformational plasticity in CA assemblies.