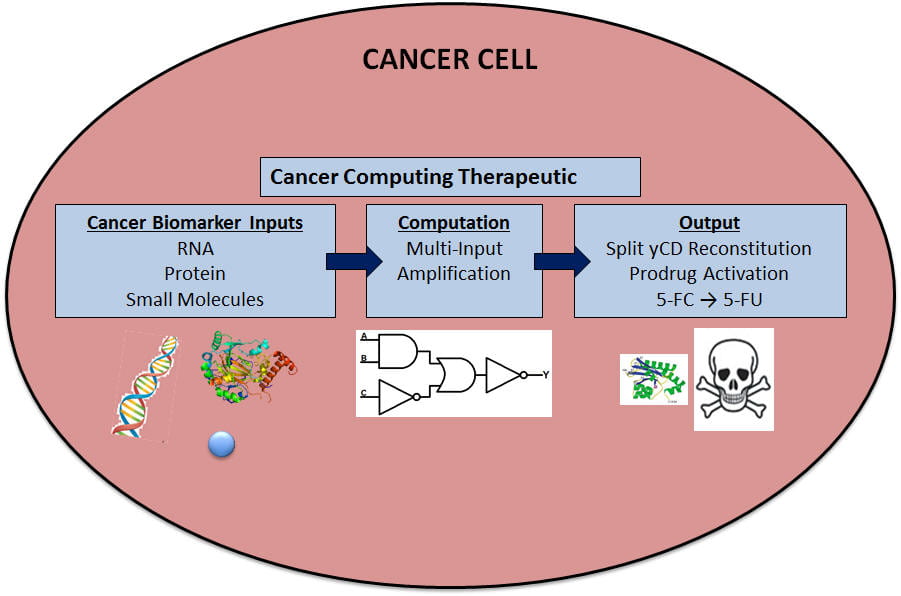
One of the most pressing needs in cancer treatment is to distinguish and treat cancer vs healthy cells. Active targeting of surface markers alone is inadequate and must be merged with additional layers of intracellular signals to provide a higher level of specificity. Advances in synthetic biology have enabled the engineering of new cellular sensors, actuators, and amplifiers toward the creation of clinically relevant “smart drugs” that sense the disease state in a complex cellular environment and actuate an appropriate, localized therapeutic response for treatment. Prior efforts have primarily been focused on implementing therapeutic gene circuits for targeted cancer therapies. However, practical utility of these synthetic devices is limited as multiple genes must be delivered and expressed within the target cell to produce needed detection components. Less complex DNA-based logic devices can potentially bypass this stringent delivery hurdle but the few successful examples reported so far can only be executed in vitro due to the need for a restriction enzyme as part of the activation circuit. Dynamic DNA-gated lock and key devices based on toehold-mediated strand displacement is a new powerful method to create intracellular logic circuits that can be triggered by cancer-specific biomarkers. By combining these dynamic DNA devices with active extracellular targeting and exogenously applied, inactive prodrugs to trigger cell death, a higher degree of specificity for cancer cells can be achieved by creating these multi-layer targeting, sensing, and responsive DNA-based devices. In this proposal, we seek to develop a new generation of synthetic DNA devices based on toehold-mediated strand displacement that can be used for programmable intracellular reconstitution of the yeast cytosine deaminase (yCD), a split suicide enzyme capable of activating the prodrug deaminate 5-fluorocytosine (5-FC) into the toxic product 5-fluorouracil (5-FU). Although the simplicity of the design allows highly adaptable, multi-input capabilities suitable for a range of cancer targets, the feasibility of the approach will be illustrated with a two-input device using two microRNAs (miRNAs) targets, miR-720 and miR-1380, associated with highly invasive inflammatory breast cancer (IBC) cells, as inputs.
Project 1. Synthetic multilayer targeting DNA devices for detection of specific cancer indicators and programmed assembly of split yCD for prodrug activation (NSF)