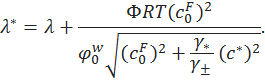Cartilage Indentation
Indentation Test for Articular Cartilage
Edited by Xingyu Chen, Van C. Mow, X. Lucas Lu.
This webpage is still under construction and will be constantly updated.
This page provides some basic information about cartilage indentation testing and the theoretical analysis of experimental data to extract the tissue properties. All the program codes are developed by us based on corresponding journal publications and free for download. If you have any question about the usage of the programs, please feel free to contact us at xlu@udel.edu.
Content:
I. Introduction to indentation testing
II. Indentation testing experiment setup
III. Analysis of the indentation
IV. Shortening the testing time
V. Estimate the GAG content with indentation testing
I. Introduction to indentation testing
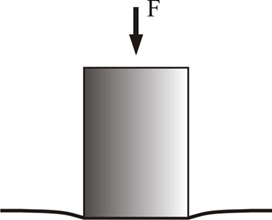
Indentation testing is a widely used technique to measure the mechanical properties of articular cartilage. A schematic diagram of an articular cartilage indentation test is shown in Figure A. Indentation is attractive because it does not require special specimen preparation such as microtoming a thin strip for tensile tests or preparing perfect cylindrical plugs for unconfined compression test. For indentation, the cartilage tissue is left untouched on the subchondral bone so that the material properties of the cartilage are determined in situ, a condition more closely resembling the physiological situation. Due to the presence of pre-stress in cartilage, stripping the tissue off from the bone may change the shape and properties of the tissue. In addition, indentation could be the only technique for the mechanical testing of cartilage on small joints, such as the temporomandibular joint (TMJ) or mouse joints, where the cartilage tissue is too thin to be harvested for other testing methods.
Indentation testing cannot directly generate the mechanical properties and this disadvantage is mainly caused by the complex boundary conditions. The interface of the indenter tip and cartilage, cartilage surface outside indenter tip, interface of cartilage and subchondral bone correspond to totally different boundary conditions in theoretical analysis. This makes the indentation problem challenging to solve, especially when the constitutive model is complicated. Only until 1972, Hayes et al. generated the first indentation solution based on a linear elastic model for cartilage with finite thickness (i.e., not an infinite half space). On this webpage, we introduce a few widely used indentation solutions and provide the corresponding programs for download.
Figure A: schematic diagram of indentation testing showing a cartilage bone block, indented by a circular, rigid, porous-permeable, and frictionless indenter tip
II. Indentation testing experiment setup
1. Creep test or stress relaxation
Mechanical testing can often be separated into two categories, load control testing and displacement control testing. In a load control test, the mechanical loading on the sample is controlled by a loading device, while the deformation history of the tissue is recorded by a displacement transducer. In the displacement control testing, the deformation of the tissue is controlled by the motor in the loading device, while the force response of the tissue is recorded by a load cell. Most commercial mechanical testing devices are equipped with a controlled motor, a displacement transducer, and a load cell. Therefore, it is natural for the device to perform displacement control testing. To perform load control testing, a feedback control program is usually required. Accuracy of the force curve is highly dependent on the dynamic performance of the motor.
1.1 Creep test
The most common load control indentation test is called the creep test, in which a step force is applied (stepwise) onto the cartilage surface while the creep deformation of the tissue is recorded (Figure B). The step loading was originally selected for indentation because the Laplace transform of a step function is easy to handle. One of the best mechanisms to apply a step loading is to drop a controlled weight on the indenter tip. As easy it sounds, a device to perform such function could be quite complicated and difficult to build. Another choice is to use a special motor that can apply the desired force transiently. Using a special motor, a custom-built device was available in our lab for the creep test. If you are interested in building a similar device, we can share the design, parts number and Labview program.
It is challenging for a displacement control device to achieve a step loading. Although most commercial loading devices have terrific dynamic responses, overshooting is almost inevitable in the force curve. In most cases, the loading speed has to be reduced to avoid too much overshooting, with the cost of a ramp load instead of a step load. The accuracy of displacement measurement must meet the accuracy requirement. Assuming the cartilage thickness is 1 mm, 10% deformation means 100 micron total displacement. Thus a device with 1 micron step size only guarantees 1% accuracy.
1.2 Stress relaxation test
In a stress relaxation test, a displacement is applied on the tissue at a constant rate until a desired level of deformation is reached. This displacement results in a force rise followed by a period of stress relaxation until an equilibrium force value is reached. The rising phase of the force curve could be challenging for a linear theory to fit. If the equilibrium stiffness of cartilage is needed, stress relaxation test could be convenient, and it generally reaches equilibrium faster than the creep test.
Figure B: Creep and stress relaxation test force and deformation profile
1.3 Dynamic indentation test
Dynamic indentation test applies sinusoidal strain on the tissue. The force response from the tissue will eventually become sinusoidal on the same frequency of the applied deformation with a certain amount of phase lag. During the test, the indenter is first controlled to apply a relatively large strain (10-20% of thickness) on the tissue, and then a small-magnitude sinusoidal displacement is applied. Measurements of the force on the indenter are recorded over time. When loading frequency is high enough, the tissue may not be able to follow the movement of indenter tip, which should be avoided.
2. Experimental setup
2.1 Sample preparation
A cartilage bone block or full joint can be used for indentation test. The cartilage size (shortest length scale) should be larger than 4 times the indenter diameter. During the test, the distance between the edges of the indenter tip and cartilage should be at least four times the indenter radius.
2.2 Sample fixation
During the entire test, cartilage should be submerged in the PBS solution or culture medium inside the loading chamber with desired ambient concentration (Figure C). Super glue is often used to fix the subchondral bone to the chamber. If the test takes longer than 0.5 hour, protease inhibitor cocktail (PI: EDTA, 2mM; benzamidine, 5mM; N-ethyl-maleiminde, 7.18 mM; and PMSF, 1.39mM) should be added into the PBS solution.
2.3 Indenter tip
The indenter tip could be flat-end or spherical-end. The theoretical analysis for the latter case is much more complicated as the contact area changes with tissue deformation. However, a flat-end tip could damage the tissue at the edge due to stress concentration. The tip could be porous or impervious to water. A porous steel tip is difficult to manufacture, but it requires less time to reach equilibrium. If a porous tip were used, it should be ultrasonically cleaned prior to the testing to ensure the ease of fluid flow inside. A “pin-technique” can be used to ensure the indenter tip is perpendicular to the cartilage surface. A small cylindrical pin is placed on the desired testing spot on the cartilage surface. The ball head under the chamber is adjusted to align the pin and the indenter tip in one axis from at least two different views. The ball head itself is usually fixed on a two-way stage.
Figure C: The ball head is adjusted to align the pin and the indenter tip to ensure the perpendicularity of the indenter tip and the cartilage surface.
2.4 Loading device
Most commercial loading devices with a proper load cell can be used for indentation testing. Custom-built devices are also commonly used. For cartilage with a few mm in thickness, the precision of the motor should be close to 1 micron.
3. Thickness measurement
In later theoretical analysis, cartilage thickness is necessary to obtain the mechanical properties. A traditional thickness measurement method is similar to indentation testing. The difference is that instead of using an indenter tip, a sharp needle is used to poke through the cartilage with a constant displacement rate until the calcified zone is reached. The displacement of the needle from touching the surface to entering the calcified zone is the thickness of the cartilage. Both of the points are indicated by a steep increase in the reaction force curve. A typical reaction force recorded during this process is shown in the figure D.
Figure D. The reaction force curve on the poking needle and the calculation of cartilage thickness.
III. Analysis of the indentation
1. Hertzian solution
The solution for indentation on a linear elastic half-space is given by the classical contact mechanics.
Figure E: Flat-end cylindrical indenter and spherical indenter applied on infinite half-space.(Picture from Wikipedia)
1.1 Spherical indenter
The applied force F on the indenter is related to the displacement d by![]() where R is the radius of the indenter, and E* is given by
where R is the radius of the indenter, and E* is given by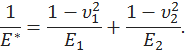 Here E1, E2, ν1, ν2 are the elastic moduli and Poisson’s ratios of the indenter and the cartilage respectively.
Here E1, E2, ν1, ν2 are the elastic moduli and Poisson’s ratios of the indenter and the cartilage respectively.
1.2 Flat-end cylinder elastic indenter
With notations in the previous section, the reaction force on the cylindrical indenter is given by![]() If incompressibility of the cartilage (ν2 = 0.5) and rigidity of the indenter (E1 → ∞) are assumed [1], the equation reduces to
If incompressibility of the cartilage (ν2 = 0.5) and rigidity of the indenter (E1 → ∞) are assumed [1], the equation reduces to![]()
2. Hayes’ solution
When the size of the indenter tip is at the same scale of cartilage thickness, the infinite half-space assumption becomes inappropriate. The indentation solution on a layered linear elastic material is given by [2]. Following the notations used previously, for a rigid indenter tip, Young’s modulus of the cartilage can be written as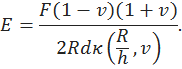
Here h is the thickness of the cartilage, and κ is an integration function associated with the shape of the indenter tip. To determine the Young’s modulus, we need equilibrium force F, equilibrium deformation d, indenter tip radius R, cartilage thickness h, and the Poisson’s ratio of cartilage. The Poisson’s ratio can be assumed with a value between 0-0.5. For articular cartilage in knee joints, a reasonable estimation is 0.2-0.3. We implemented the Hayes’ solution in MATLAB, for both spherical and flat-end cylindrical indenter, and the code can be downloaded here.
3. Linear and nonlinear poroelastic models
As a mixture of solid matrix and fluid, cartilage is a typical poroelastic material. Under compressive loading, the fluid component within the tissue may flow out of the tissue, like water being squeezed out of a sponge. The ease with which fluid may flow through a porous solid matrix is a measure of its permeability. The lower permeability (usually associated with smaller pores), the more difficult it is to force fluid through the solid matrix; thus the slower it takes the tissue to reach final equilibrium state under loading. Flow-dependent viscosity contributes significantly to the viscoelastic behavior of cartilage under indentation, i.e., the creep or stress relaxation curves.
Figure F: An indentation creep curve fitted by nonlinear (left)/linear (right) elastic biphasic models.
3.1 Poroelastic (biphasic) model
In the simplest case, linear elasticity is assumed for the solid matrix. Such model is termed the biphasic linear elastic (BLE) model. In this model, only three material properties are necessary to characterize the cartilage: Young’s modulus E, Poisson’s ratio v and hydraulic permeability k. At final steady state, the behavior of BLE material is the same with linear elastic material, thus the Hayes’ solution is still valid for the analysis of equilibrium data. Mow et al. [3] have developed a curve-fitting program which can uniquely determine all three material properties using a single indentation creep test. To use this program, a creep test with a constant step loading has to be performed with a flat-end cylindrical porous indenter tip. The full creep curve has to be recorded and input into the program. A sample of the program can be downloaded here.
3.2 Biphasic tension-compression nonlinear model
Cartilage is experimentally shown to have one order of magnitude difference in terms of the modulus under compression and tension. Such difference has significant impact on the response of the cartilage under indentation. For example, at the very beginning of the creep test, according to the biphasic theory, the material is almost incompressible, thus the tensile modulus in lateral directions can directly contribute to the stiffness in loading direction. Many models are available to catch this nonlinearity in tension and compression. Among them, the conewise linear elastic (CLE) model and continuous fiber distribution (CFD) model are commonly used [4][5]. In addition to the BLE model, a new material property tensile modulus is introduced for the solid matrix (in BLE model, the tensile and compressive moduli are the same). We built the indentation models on these two constitutive assumptions in FEBio, and the curve fitting program has been developed to determine the compressive, tensile moduli and the permeability through a single indentation creep curve. The curve-fitting program can be downloaded here.  Figure G: Finite element mesh for nonlinear indentation analysis.
Figure G: Finite element mesh for nonlinear indentation analysis.
IV. Shortening the testing time
Indentation creep tests could take hours to reach; and the actual time is related to cartilage thickness, indenter size, tissue permeability and stiffness. The lengthy testing time reduces the efficiency of indentation, increases the chance of tissue deterioration, and prevents testing at multiple regions. To overcome these limitations, principal component analysis (PCA) can be used to predict the full indentation creep curve based on the deformation history of the first few minutes and the principal components. Brief steps of doing this prediction is introduced here and details can be found in [6].
1. Generating principal component (PC) matrix
Full tests should be conducted on a few samples (N ≥ 5) first. PCA is then conducted on the deformation history to extract the principal components.
2. Prediction of full creep curve
Figure H: Typical creep curve predictions (left) and mechanical properties extracted from predicted and original creep curves for different regions on TMJ (right)
The short-term response (5 to 10 minutes) of the samples in the testing group is projected onto the space spanned by the principal components of the full tests group. It is assumed that the long-term response of the samples will be projected onto the PC space with the same set of coefficients as the short-time response, and thus the long-term response is estimated. The principal analysis code can be downloaded here.
V. Estimate the GAG content with indentation testing
A major compositional factor, one that has received intense biochemical and physiochemical scrutiny during the past several decades, is the charged nature of glycosaminoglycan (GAG) in cartilage. These negative charges give rise to a high charge density within the tissue which is commonly known in the literature as the fixed charge density (FCD). Because each fixed negative charge requires a mobile counter-ion (e.g. Na+) to maintain the electro-neutrality within the interstitium, FCD gives rise to an imbalance of mobile ions between interstitium and the external bathing solutions. This excess of mobile ions yields a pressure difference between the internal and external aqueous solutions, which is widely known as the Donnan osmotic pressure. This osmotic effect can profoundly affect tissue hydration, control of fluid content, ion transport, and a board spectrum of other observed mechanical responses. For example, apparent stiffness of cartilage can be significantly different in hypertonic and isotonic solution. Indentation tests conducted on the same sample in different ambient solution concentrations are shown below.
Figure I. A set of typical indentation creep curves of articular cartilage bathed in 2M and 0.15M PBS solutions.
If the solid matrix is assumed to be linear elastic, the relation between the apparent mechanical properties of cartilage and the intrinsic mechanical properties is revealed by the correspondence principle [7]. The equation relating the intrinsic mechanical properties (solid matrix true mechanical properties) and the apparent mechanical properties (mechanical properties involving the osmotic effect) are given by
Here μ*, λ*, and μ, λ are the Lame constants of apparent and intrinsic mechanical properties respectively. Φ is the osmotic coefficient of the solution, R is the universal gas constant and T is the absolute temperature. c0F and φ0w are the FCD and water volumetric fraction at the reference configuration respectively. c* is the concentration of the outside bathing solution, and γ* and γ± are the activity coefficients of the ions within the bath and within the pores respectively. To use this correspondence principle, two mechanical tests can be performed with different solution concentrations, and the intrinsic and apparent mechanical properties can be obtained with Hayes’ solution or biphasic linear model. With both intrinsic and apparent mechanical properties, the FCD of the tissue can be calculated using the above equations. The FCD value can be used to estimate the GAG content of the tissue.
Figure J. Validation of the calculated FCD values from correspondence principle with those from biochemical GAG assay
References
Download
Hayes solution for indentation analysis (Hayes1972, LuMow2008)
Download
Indentation curve-fitting program based on linear biphasic theory (Mow 1989, Lu 2004)
Download
Indentation curve-fitting program to determine the tension-compression nonlinear properties of cartilage (Chen, 2015, Ruggiero 2015).
Download
Principal analysis of indentation creep curve (Chen et al., 2015).