Water is important for all life forms on planet Earth. Water is in our bodies, and is used in every part of our lives. But water can also contain contaminants that make it non-potable, not healthy for us to drink. Contaminants can be:
Physical: particles of soil or organic matter from soil erosion
Chemical: elements or compounds that are natural or human-made, such as pesticides, bleach, nitrogen, human and/or animal drugs, metals, or toxins produced by bacteria. Some chemical contaminants (such as cesium, plutonium and uranium) are also dangerous because they can emit radiation.
Biological (or Microbial): organisms that live in water, such as bacteria, viruses, protozoan, and parasites
Non-potable water is also called grey water. So how do we remove contaminants from grey water to make it potable, or safe to drink?
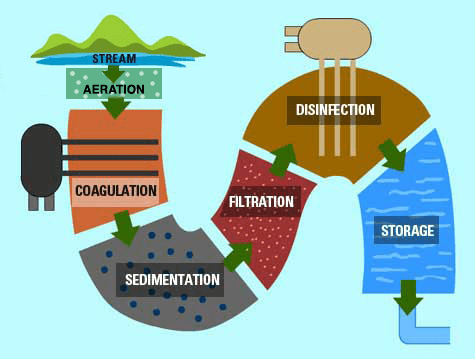
Source: http://www.eschooltoday.com/global-water-scarcity/how-water-is-treated-for-drinking.html
This graphic shows a basic water treatment process for surface water. The water from a stream enters the Water Treatment Center and goes through the following processes:
Aeration: Often the first process at a Water Treatment Center, the water is brought into close contact with air. This removes dissolved gases, oxidizes dissolved metals (which makes them fall out of solution and become particles), and volatile organic compounds (VOCs).
Coagulation: Alum and other chemicals are added to the water. This causes particles to chemically stick together into floc.
Sedimentation: When the particles of floc get big enough, gravity causes them to sink to the bottom.
Filtration: The process of removing remaining small impurities.
Disinfection: Chlorine or other kinds of disinfection methods are applied to kill any bacteria and other living organisms that may be in the water.
Storage: Water is stored in tanks while the process of disinfection completes, and then is transported through pipes to our homes and workplaces
For more information about Wastewater Treatment Processes:
Treating Dirty Water
CAUTION: In this experiment we will process “dirty water” using techniques that are used in water treatment facilities. Although experiment illustrates how water is cleaned, the water processed will not be cleaned to the extent of being potable. FOR YOUR SAFETY, DO NOT DRINK ANY WATER PROCESSED IN THIS EXPERIMENT.
Materials
For Safety and Tidiness
- Safety glasses
- Cover for clothing (old shirt, apron, or smock)
- Table cover
- Paper towels
To Make “Grey Water” (Non-Potable Water)
- Liter- or quart-sized container with a lid (an empty 2-liter bottle works well)
- Two large cups (to pour liquid back and forth)
- Salad dressing (Italian works well, due to oiliness and suspended organic particles)
- Dirt (a handful or so)
- 1 L (approximately) Tap Water
- Liter- or quart-sized container with a lid (an empty 2-L bottle works well)
- Two large cups (to pour liquid back and forth)
- 1 C (approximate) Salad dressing (Italian works well, due to oiliness and suspended organic particles)
For Water Treatment Processes
- 1 plastic 2-L bottle (cut with scissors into two parts:
- 1 Tbsp of alum (found in the spice or canning section of the supermarket)
- Stirring spoon
- Clear Tupperware container or large cup (to filter into)
- 2 rubber bands
- 2–5 coffee filters
- Variety of materials to use as filter media (e.g., cotton balls, aquarium gravel, play sand, activated carbon, pebbles, marbles, etc.)
- Clean tap water (a pitcher works well)
- 2 litmus color strips and a pH color chart (optional)
- Digital multimeter (optional)
Instructions
- Make the Grey Water: Mix a liter or so of water with a cup of salad dressing and a handful or so of dirt and place in the container with a cap.
Aeration
- Cap the container and shake the bottle to aerate the contents for a minute or two.
- Pour the dirty water into the first large cup and then pour it from one large cup to the other to further aerate it.
Coagulation
- Pour the aerated dirty water into the bottom half of the 2-liter bottle that has been cut in half.
- Add 1 Tbsp Alum to the aerated dirty water.
- Slowly stir the mixture for 5 minutes. You will begin to see particles forming in the liquid; this is the floc.
Sedimentation
- Allow the dirty water to stand untouched for 20 minutes. The particles will sink to the bottom of the container. Meanwhile, make your filter for the next step.
Filtration
- Making your filter:
- Place one or two coffee filters (depending on how strong/likely to rip they seem) over the mouth of the top half of the 2-liter bottle that was cut in half. Make sure the coffee filters cover the whole opening (the smaller end where the cap would go; you don’t need the cap) and secure them with the rubber bands.
- Use your materials to design your filter. You will want to have 3 distinct layers for the dirty water to pass through. Depending on what materials you have, think about what might be the best combination and best order to layer materials in order to remove the most impurities. You can plan your filter using this diagram:
![Diagram of top half of two-liter soda bottle, with the large opening on the top and the mouth of the bottle on the bottom. The mouth of the bottle has a coffee filter secured to it with a rubber band, blocking the opening completely. Three layers are demarcated for labeling to indicate which material is planned for which layer of the filter.]](https://sites.udel.edu/k12engineering/files/2020/05/filterlevels.jpg)
- Place your filter in the container into which you will be filtering. Arrange your layers of material. Without disturbing the top layer, pour clean tap water through your filter. After the filtered water collects in the filtering container, record your observations on the Filtration Experiment Data Sheet.
You can write down your observations about the odor and appearance of the filtered clean water in the boxes. If you have litmus paper and/or a multimeter, you can record the pH and electrical conductivity of the filtered clean water as well. Gently move your filter and keep it upright when you empty your filtering container, so as not to disturb the layers of filtering material, after you have finished making and recording observations. - Leaving the sediment that has separated from the dirty water at the bottom of the container, pour as much of the dirty water into a new container (one of the large cups used in the aeration step, rinsed, would be fine).
Write down your observations about the odor and appearance of the dirty water in the boxes on the Filtration Experiment Data Sheet. If you have litmus paper and/or a multimeter, you can record the pH and electrical conductivity of the dirty water as well. - Place your filter back into the container into which you will be filtering. Without disturbing the top layer, pour the dirty water through your filter. After the filtered water collects in the filtering container, record your observations on the Filtration Experiment Data Sheet.
Gently move your filter and keep it upright when you pour the contents of the filtering container into another container (one of the large cups used in the aeration step, rinsed, would be fine), after you have finished making and recording observations. - Place your filter back into the container into which you will be filtering. Without disturbing the top layer, pour the once-filtered dirty water through your filter. After the twice-filtered water collects in the filtering container, record your observations on the Filtration Experiment Data Sheet.
- Review your observations and consider the follow-up questions on the Filtration Experiment Data Sheet.
UD Connection
Engineering students at the University of Delaware’s chapter of Engineers without Borders (EWB) have worked with communities all over the world to improve their drinking water. Using the skills they have learned in classes and on-site training, UD EWB has built filters and pumps for water in Malawi and the Philippines. This is just one example of how engineers use science to solve problems.
©2020 UD K-12 Engineering

